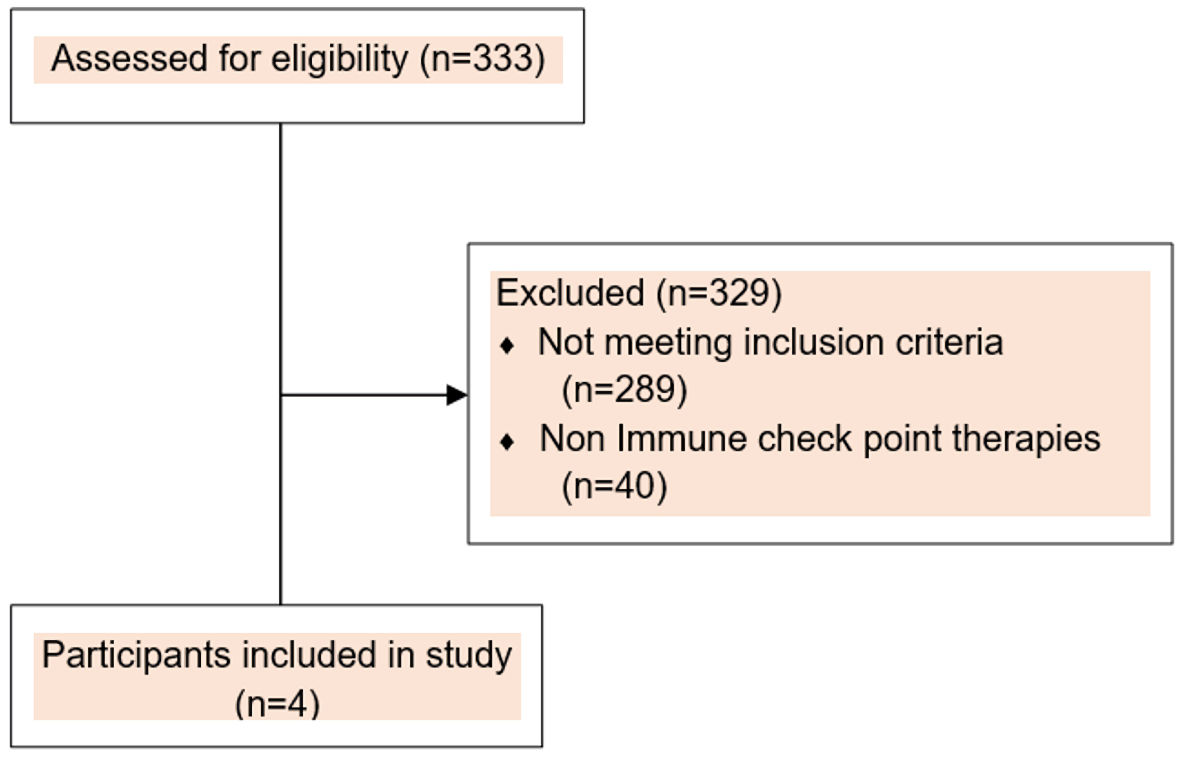
Figure 1. Consort flow diagram.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 3, June 2022, pages 113-120
Spectrum of Immune Checkpoint Inhibitor Anemias: Results From a Single Center, Early-Phase Clinical Trials Case Series Experience
Figure

Tables
| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| aFrom last dose of ICI. ICI: immune checkpoint inhibitor; ICI-A: immune checkpoint inhibitor anemia; ECOG: Eastern Cooperative Oncology Group; CLL: chronic lymphocytic leukemia; heme-irAEs: hematological immune-related adverse events; PD-L1: programmed cell death ligand 1; PD-1: programmed cell death protein 1; PARP: poly-(ADP ribose) polymerase; ICOS: inducible T-cell co-stimulator; VEGF: vascular endothelial growth factor; IL: interleukin. | ||||
| Age | 69 | 68 | 65 | 68 |
| ECOG | 2 | 1 | 0 | 0 |
| Sex | M | F | F | F |
| Cancer type | Non-small cell lung cancer | Gastroesophageal cancer | Esophageal cancer | Renal cell carcinoma |
| Histology | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Clear cell carcinoma |
| Prior lines of therapy | 1. cisplatin + pemetrexed 2. carboplatin + pemetrexed 3. single agent pemetrexed | 1. mFOLFOX | 1. mFOLFOX 2. paclitaxel + ramucirumab | 1. sunitinib |
| Comorbidities | CLL | Hypothyroidism | Anxiety | Diabetes mellitus |
| Genomic alterations | KRAS_G12F KRAS_K167E ERBB4_R306C STK11_A241P TP53_R249S PDL1 0% | HER2 negative PDL1 10-20% MMR intact Germline BRCA2 mutation: (BRCA2):c.1813dup (p.Ile605fs) | EGFR_Q217K CDKN2A_M52V MMR intact PDL1 5% HER2 negative | N/A |
| Prior ICI exposure | No | No | No | No |
| Mechanism of action | STAT3 + anti-PD-L1 monoclonal antibody | PARP inhibitor + anti-PD-1 monoclonal antibody + VEGF inhibitor | ICOS agonist monoclonal antibody + anti-PD-1 monoclonal antibody | Pegylated recombinant IL-10 + anti-PD-1 monoclonal antibody |
| Time to ICI-Aa | 4 weeks | 6 weeks | 3 weeks | 2 weeks |
| Time to recovery | 20 weeks | 8 weeks | 0 weeks | 4 weeks |
| No. of cycle pre-ICI-A | 11 | 21 | 2 | 4 |
| No. of cycles post-ICI-A | 0 | 14 | 0 | 0 |
| Other heme-irAEs | Thrombocytopenia | No | Thrombocytopenia | Pancytopenia |
| Case | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| ICI-A: immune checkpoint inhibitor anemia; DAT: direct antiglobulin test; LDH: lactate dehydrogenase: N/A: not available; IgG: immunoglobulin G. | |||||
| Hemoglobin | Pre | 8.9 | 11.8 | 13.5 | 13.9 |
| Nadir | 4.6 | 7.8 | 5.9 | 7.7 | |
| Post | 10.2 | 10.8 | N/A | 13 | |
| LDH | Pre | 319 | 206 | 499 | 346 |
| Nadir | 439 | 259 | 7,175 | 4,896 | |
| Post | 315 | 228 | N/A | 424 | |
| Reticulocyte count, % | Pre | N/A | N/A | N/A | 1.1 |
| Nadir | 1.2 | 1.9 | 14.4 | 3.9 | |
| Post | 1.2 | N/A | N/A | 2.4 | |
| Haptoglobin | Pre | 131 | N/A | N/A | N/A |
| Nadir | < 3 | 16 | < 3 | < 3 | |
| Post | 176 | 38 | N/A | < 3 | |
| Total bilirubin | Pre | 0.8 | 0.3 | 0.7 | 0.2 |
| Nadir | 1.3 | 0.6 | 7.1 | 1 | |
| Post | 0.3 | 0.3 | N/A | 0.5 | |
| Ferritin | Pre | N/A | N/A | N/A | N/A |
| Nadir | 1,074 | 770 | 1,031 | 32,110 | |
| Post | N/A | N/A | N/A | 750 | |
| Spherocytes | No | No | Yes | Yes | |
| DAT | IgG- C3+ | IgG- C3- | IgG- C3- | IgG+ C3- | |
| Case | Immunosuppressive agent | PRBC use | Rechallenge with ICI | ICI-A recurrence | Best response | PFS | OS | Further lines of therapy |
|---|---|---|---|---|---|---|---|---|
| aThe patient withdrew consent while in PR after 34 cycles and lost to follow-up. bAlive currently. QD: once a day; QW: once a week; QM: once a month; PO: orally; Q12H: every 12 h; Q3D: every 3 days; BID: twice a day; MP: methylprednisolone; PRBC: packed red blood cell; ICI-A: immune checkpoint inhibitor anemia; ICI: immune checkpoint inhibitor; PFS: progression-free survival; OS: overall survival; SD: stable disease; PR: partial response; N/A: not available. | ||||||||
| 1 | Cyclosporine 200 mg QD × 30 weeks | 20 units | No | N/A | SD (-1%) | 46 weeks | 79 weeks | Afatinib |
| Rituximab 1 g QW × 4 weeks | ||||||||
| IVIG 1 g QM × 18 weeks | ||||||||
| MP 100 - 200 mg IV × 4 doses, prednisone 60 mg PO QD tapered over 25 weeks | ||||||||
| 2 | None | 6 units | Yes | No | PR (-46%) | N/Aa | N/Aa | N/A |
| 3 | MP 1 mg/kg Q12H × 5 days | 5 units | No | N/A | N/A | 6 weeks | 6 weeks | None |
| 4 | Etoposide 100 mg/m2 Q3D × 3 doses + dexamethasone 10 mg BID tapered over 1 month + 1 dose of tocilizumab 4 mg/kg | 3 units | No | N/A | SD (0%) | 52 weeks | N/Ab | Axitinib |