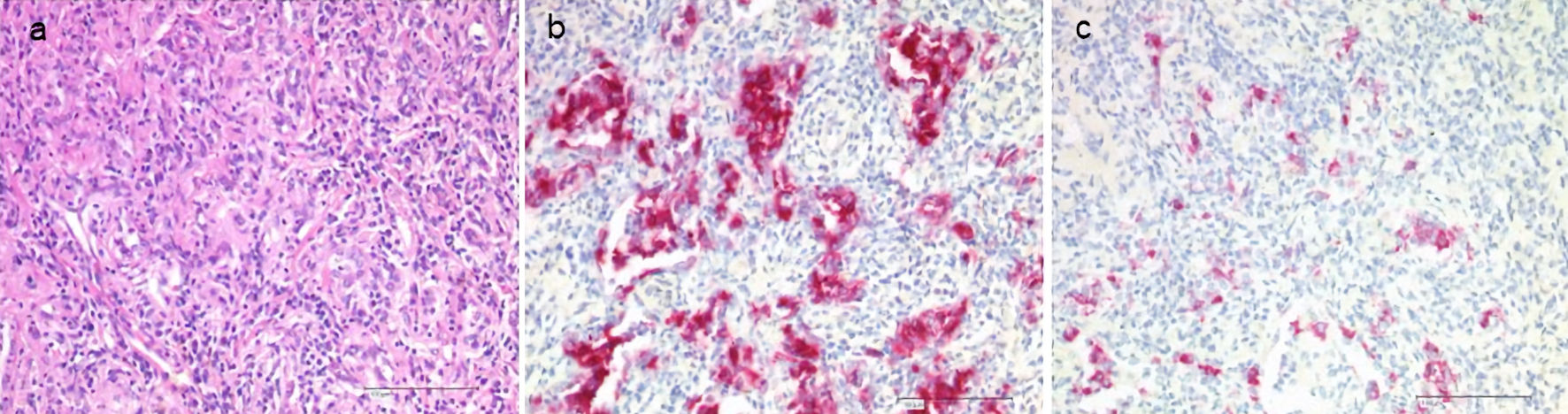
Figure 1. Histology of LCH in the lungs. Staining with hematoxylin and eosin (H&E, a), CD1a (b), CD207 (Langerin, c). LCH: Langerhans cell histiocytosis.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 11, Number 4, August 2022, pages 131-141
Experiences of a Single Center in One Hundred Ninety-Four Adult Patients With Langerhans Cell Histiocytosis
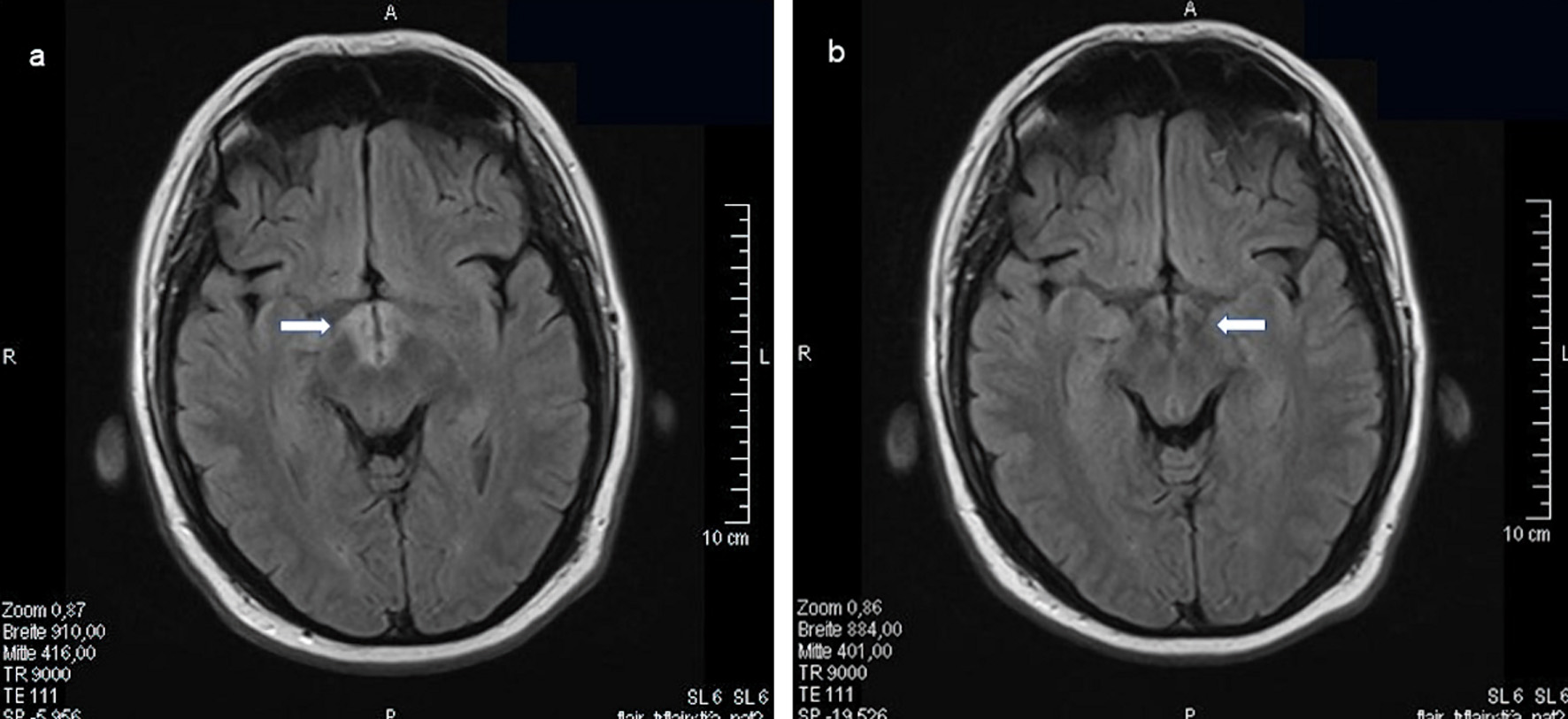
Figures

Tables
| Organs | Single system disease | Multisystem disease | Altogether |
|---|---|---|---|
| 127 patients, n (%) | 67 patients, n (%) | 194 patients, n (%) | |
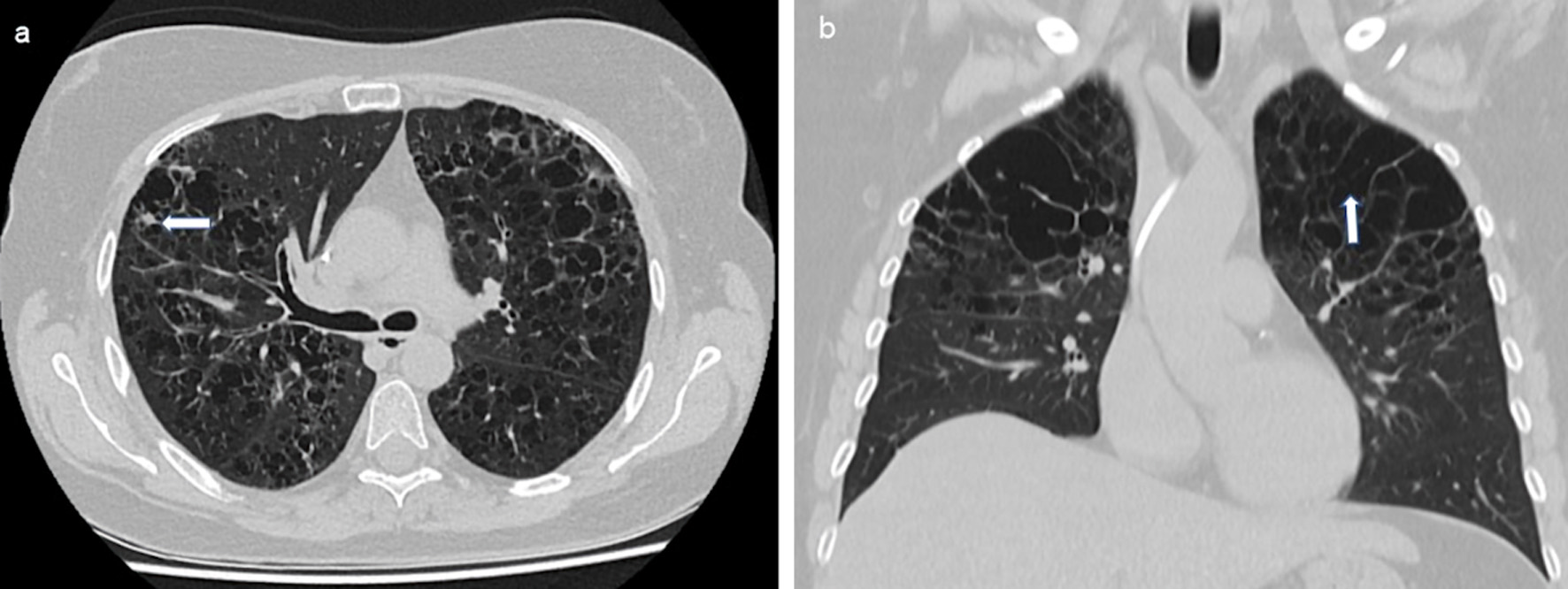
| Lungs | 39 (20.1) | 40 (20.6) | 79 (40.7) |
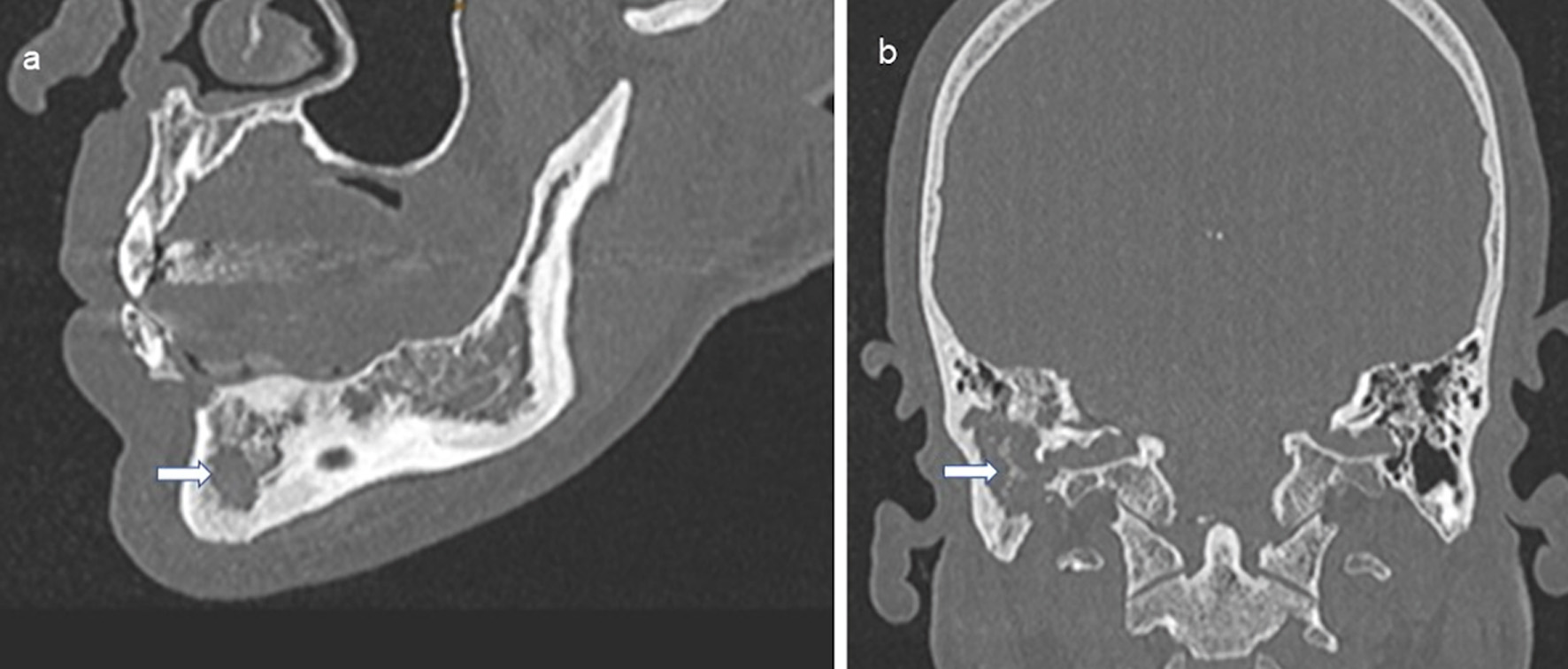
| Bones | 62 (32) | 50 (25.8) | 112 (57.7) |
| Unifocal | 44 (22.7) | 18 (9.3) | 62 (32) |
| Multifocal | 18 (9.3) | 32 (16.5) | 50 (25.8) |
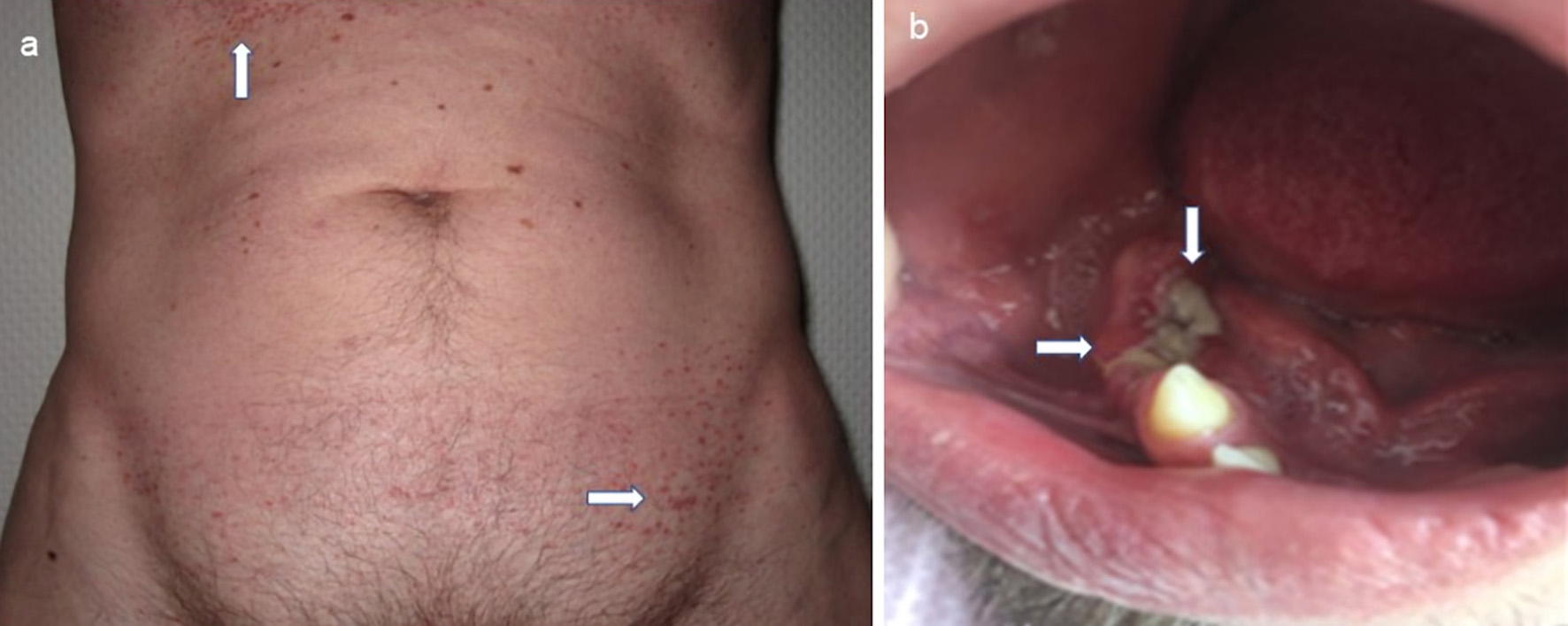
| Skin | 15 (7.7) | 19 (9.8) | 34 (17.5) |
| Unilocular | 7 (3.6) | 0 | 7 (3, 6) |
| Multilocular | 8 (4.1) | 19 (9.8) | 27 (13, 9) |
| Oral mucosa | 0 | 6 (3.1) | 6 (3.1) |
| Genital mucosa | 2 (1) | 3 (1.5) | 5 (2.6) |
| Central nervous system | 0 | 5 (2.6) | 5 (2.6) |
| Pituitary gland | 1 (0.5) | 29 (14.9) | 30 (15.5) |
| Neurohypophysis | 0 | 18 (9.3) | 18 (9.3) |
| Neuro- and adenohypophysis | 1 (0.5) | 11 (5.7) | 12 (6.2) |
| Lymph nodes | 4 (2.1) | 7 (3.6) | 11 (5.7) |
| Spleen | 0 | 1 (0.5) | 1 (0.5) |
| Liver | 0 | 7 (3.6) | 7 (3.6) |
| Gastrointestinal tract | 2 (1) | 5 (2.6) | 7 (3.6) |
| Esophagus | 0 | 2 (1) | 2 (1) |
| Stomach | 0 | 2 (1) | 2 (1) |
| Colon | 2 (1) | 1 (0.5) | 3 (1.5) |
| Sclera | 1 (0.5) | 0 | 1 (0.5) |
| Retrobulbar space | 0 | 1 (0.5) | 1 (0.5) |
| Heart | 0 | 1 (0.5) | 1 (0.5) |
| Kidneys | 1 (0.5) | 1 (0.5) | 2 (1) |
| Adrenal glands | 0 | 1 (0.5) | 1 (0.5) |
| Chemotherapy (cytostatic drugs) | Evaluable patients (cytostatic pretreated) | Organ involvement (SS, MS) | Therapy response (ORR%) | Median duration, therapy response (months) |
|---|---|---|---|---|
| MS: multisystem disease; SS: single system disease; ORR: overall remission rate; CR: complete regression; PR: partial regression; SD: stable course or mixed response. | ||||
| Cladribine | 29 (13) | SS 9 | PR 22 (76%) | 21 |
| MS 20 | SD 6 (21%) | |||
| PD 1 (3%) | ||||
| (ORR 76%) | ||||
| Cladribine plus cytarabine | 4 (1) | SS 1 | PR 3 (75%) | 39 |
| MS 3 | SD 1 (25%) | |||
| (ORR (75%) | ||||
| Cytarabine | 16 (3) | SS 3 | PR 7 (44%) | 28 |
| MS 13 | SD 5 (31%) | |||
| PD 4 (25%) | ||||
| (ORR 44%) | ||||
| Etoposide | 5 (5) | SS 1 | PR 3 (60%) | 34 |
| MS 4 | SD 2 (40%) | |||
| (ORR 60%) | ||||
| Methotrexate | 12 (4) | SS 8 | CR 1 (8%) | 17 |
| MS 4 | PR 5 (42%) | 14 | ||
| SD 5 (42%) | ||||
| PD 1 (8%) | ||||
| (ORR 50%) | ||||
| Vinblastine plus prednisone | 14 (2) | SS 6 | PR 5 (36%) | 27 |
| MS 8 | SD 9 (64%) | |||
| (ORR 36%) | ||||
| Vinblastine plus prednisone plus 6-mercaptopurine | 24 (3) | SS 8 | CR 2 (8%) | 96 |
| MS 16 | PR 8 (33%) | 30 | ||
| SD 11 (46%) | ||||
| PD 3 (12%) | ||||
| (ORR 41%) | ||||
| Vinblastine plus cyclophosphamide plus 6-mercaptopurine | 2 (0) | MS 2 | SD 2 (100%) | |
| Vinblastine plus etoposide plus 6-mercaptopruine | 2 (0) | SS 1 | SD 2 (100%) | |
| MS 1 | ||||
| Permanent consequences | Patients, n (%) |
|---|---|
| ECD: Erdheim-Chester disease; LCH: Langerhans cell histiocytosis. | |
| Endocrine dysfunction | 36 (18.6) |
| Neurohypophysis | 21 (10.8) |
| Neuro- and adenohypophysis | 15 (7.7) |
| Pulmonary dysfunction | 15 (7.7) |
| Neurological dysfunction | 9 (4.6) |
| Hearing loss and balance disorders | 6 (3.1) |
| Tooth loss | 5 (2.6) |
| Death | 4 (2.1) |
| LCH/ECD | 2 (1) |
| Therapy induced sepsis | 2 (1) |
| Blindness of one eye | 2 (1) |
| Malignant disease | 1 (0.5) |
| Liver cirrhosis | 1 (0.5) |
| Skin ulcerations | 1 (0.5) |