Figure 1. PRISMA flow chart for study selection criteria.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 11, Number 4, August 2022, pages 123-130
Treatment of Light Chain Deposition Disease: A Systematic Review
Figures

Table
| First author, year | Evaluable patients (LCDD only), n (%) | Primary treatment | Conditioning regimen if ASCT | Median age in years (range) | Hematologic response (VGPR or greater) | Biopsy-proven renal disease | Renal response, n (%) | Survival outcomes |
|---|---|---|---|---|---|---|---|---|
| LCDD: light chain deposition disease; ASCT: autologous stem cell transplant; BorD: bortezomib + dexamethasone; Dex: dexamethasone; VAD: vincristine + adriamycin + dexamethasone; VGPR: very good partial response; n: number; NA: not available; mos: months; f/u: follow-up. | ||||||||
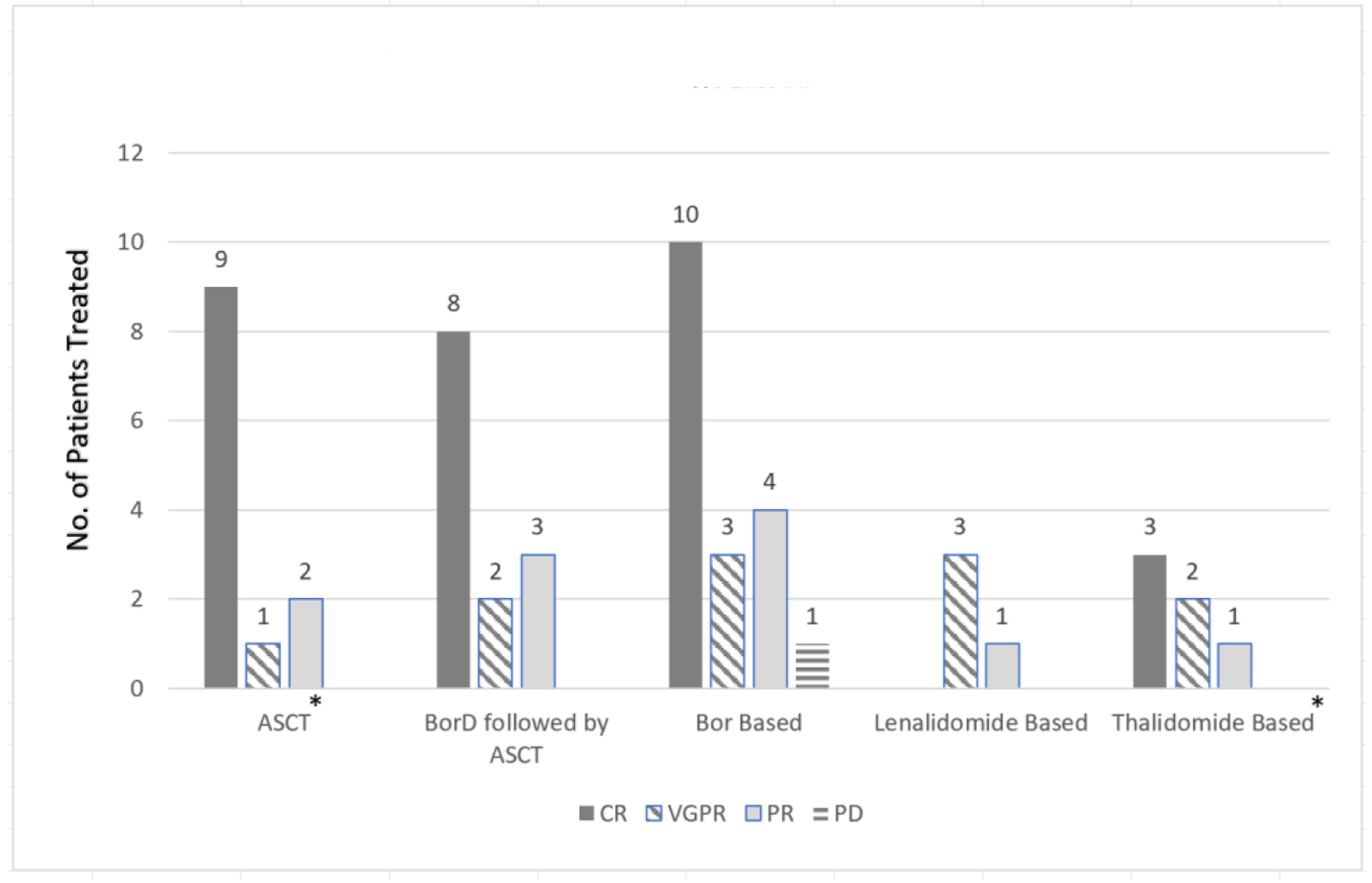
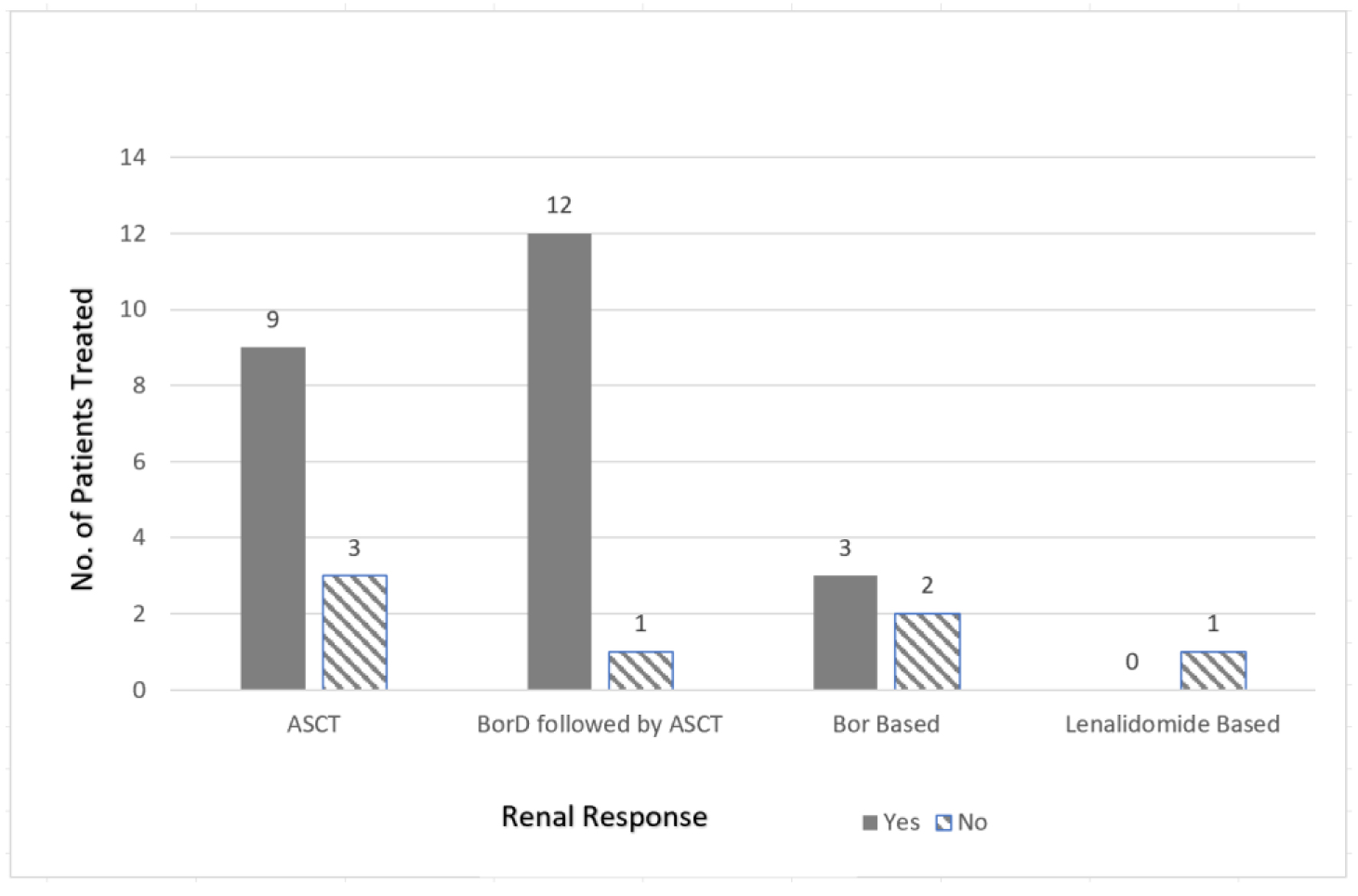
| Lorenz et al, 2008 [11] | 3/6 (50%) | Dex (67%) followed by ASCT | Melphalan 200 mg/m2 or reduced | 39 (33 - 43) | 100% | 1 (33%) | 66% alive after median f/u of 46.2 mos | |
| Kimura et al, 2018 [12] | 3/3 (100%) | Prior BorD (33%) followed by lenalidomide-based regimen | 60 (59 - 69) | 100% | 100% | 0 (0%) | ||
| Jimenez-Zapeda et al, 2012 [13] | 6/6 (100%) | BorD (50%) or Dex (50%) followed by ASCT | Melphalan 140 - 200 mg/m2 | NA | 83% | NA | Median f/u 23 mos | |
| Kastritis et al, 2009 [14] | 4/4 (100%) | Various prior (VAD or cyclophosphamide/prednisone) followed by BorD then ASCT (75%) | High-dose melphalan | 52 (46 - 56) | 67% | 100% | 3 (100%) | 100% alive after f/u of 10 - 18 mos |
| BorD only (25%) | 67 | 0% | 1 (100%) | |||||
| Lessi et al, 2012 [15] | 4/4 (100%) | BorD only (25%) | 44.5 (37 - 64) | 67% | 75% | 4 (100%) | ||
| BorD followed by ASCT (75%) | Melphalan 140 mg/m2 | 0% | ||||||
| Minarik et al, 2012 [16] | 3/3 (100%) | BorD | 48 (33 - 56) | NA | 100% | 1 (33%) | ||
| Sayed et al, 2015 [17] | 25/53 (47%) | Thalidomide based (n = 11) | 56 (29 - 78) | 45% | 100% | NA | Median f/u 74.4 mos | |
| Bortezomib based (n = 9) | 89% | |||||||
| ASCT (n = 4) | Melphalan | 100% | ||||||
| Lenalidomide based (n = 1) | 0% | |||||||
| Telio et al, 2012 [18] | 5/8 (63%) | BorD (20%) or Dex (60%) followed by ASCT | Melphalan 140 - 200 mg/m2 | 48 (40 - 55) | 20% | 100% | 4 (80%) | Median f/u 29 mos |
| Tovar et al, 2012 [19] | 3/3 (100%) | BorD followed by ASCT (100%) | Melphalan 140 - 200 mg/m2 | 48 (36 - 63) | 100% | 100% | 2 (67%) | 100% alive after a median f/u of 34 mos |
| Weichman et al, 2006 [20] | 4/6 (67%) | ASCT | Melphalan 140 - 200 mg/m2 | 45 (36 - 51) | 100% | 100% | 2 (50%) | 100% alive after a median f/u of 12 mos |
| Hassan Zafar et al, 2011 [21] | 4/20 (20%) | BorD | NA | 25% | 100% | |||
| Kastritis et al, 2021 [22] | 6/25 | Daratumumab | 67.5 (59 - 83) | 67% | Median f/u 25 mos | |||