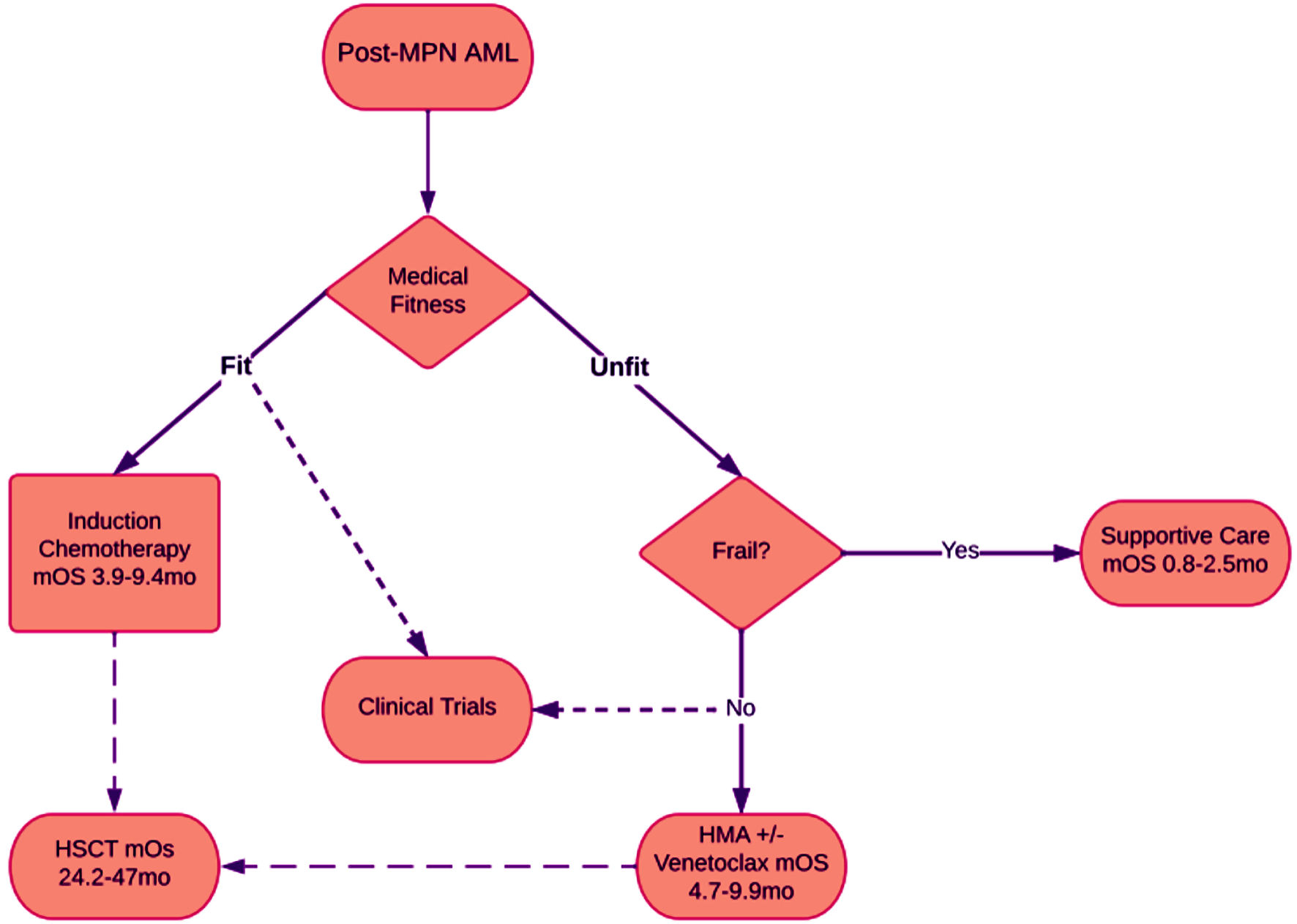
| Induction chemotherapy | | | |
| Mesa et al, 2005 [10] | 24 | 3.9 | Cytarabine, mitoxantrone, gemtuzumab |
| Tam et al, 2008 [48] | 36 | 6 | |
| Passamonti et al, 2010 [4] | 8 | 5.6 | |
| Kennedy et al, 2013 [49] | 13 | 9.4 | |
| Badar et al, 2015 [50] | 35 | 7.6 | No significant difference between induction and decitabine |
| Venton et al, 2017 [51] | 34 | 8.3 | |
| Lancet et al, 2021 [47] | 156 | 5.95 | |
| Lancet et al, 2021 [47] | 153 | 9.33 | Liposomal daunorubicin and cytarabine |
| Gangat et al, 2021 [52] | 69 | 8 | |
| Castillo Tokumori et al, 2022 [53] | 28 | 11.4 | This number is higher due to HSCT after chemotherapy |
| Castillo Tokumori et al, 2022 [53] | 15 | 4.9 | Chemotherapy alone |
| Non-induction chemotherapy | | | |
| Mesa et al, 2005 [10] | 48 | 2.9 | Vincristine, melphalan, cytarabine (low-dose), etoposide, etc. |
| Tam et al, 2008 [48] | 12 | 7 | |
| Passamonti et al, 2010 [4] | 8 | 2.5 | |
| HMA +/- venetoclax | | | |
| Thepot et al, 2010 [54] | 26 | 8 | Azacytidine |
| Kennedy et al, 2013 [49] | 15 | 6.6 | |
| Andriani et al, 2015 [55] | 19 | 9.9 | Azacytidine |
| Badar et al, 2015 [50] | 21 | 6.9 | Decitabine |
| Venton et al, 2017 [51] | 11 | 7.9 | Azacytidine |
| Mascarenhas et al, 2020 [56] | 25 | 9.5 | Decitabine and ruxolitinib |
| Gangat et al, 2021 [52] | 32 | 8 | |
| Gangat et al, 2021 [52] | 26 | 5.5 | HMA + venetoclax |
| Castillo Tokumori et al, 2022 [53] | 28 | 4.7 | HMA alone |
| Supportive care | | | |
| Mesa et al, 2005 [10] | 19 | 2 | |
| Tam et al, 2008 [48] | 19 | 1.5 | |
| Passamonti et al, 2010 [4] | 7 | 2.5 | |
| Venton et al, 2017 [51] | 28 | 1.8 | |
| Castillo Tokumori et al, 2022 [53] | 19 | 0.8 | |
| Stem cell transplant | | | |
| Tam et al, 2008 [48] | 8 | | 73% survival at 31months follow-up |
| Passamonti et al, 2010 [4] | 1 | | > 70-day survival |
| Cherington et al, 2012 [9] | 8 | | PFS 74.9% at 2 years |
| Kennedy et al, 2013 [49] | 17 | 47 | |
| Badar et al, 2015 [50] | 7 | | 4/7 died in the first 100 days |
| Venton et al, 2017 [51] | 9 | 24.2 | |
| Gangat et al, 2021 [52] | 6 | | Received HSCT after venetoclax + HMA |
| Castillo Tokumori et al, 2022 [53] | 13 | 40.8 | |
| All patients with LT of MPN | | | |
| Mesa et al, 2005 [10] | 91 | 2.7 | |
| Tam et al, 2008 [48] | 74 | 5 | |
| Tefferi et al, 2017 [44] | 410 | 3.6 | No improvement in the last 15 years |