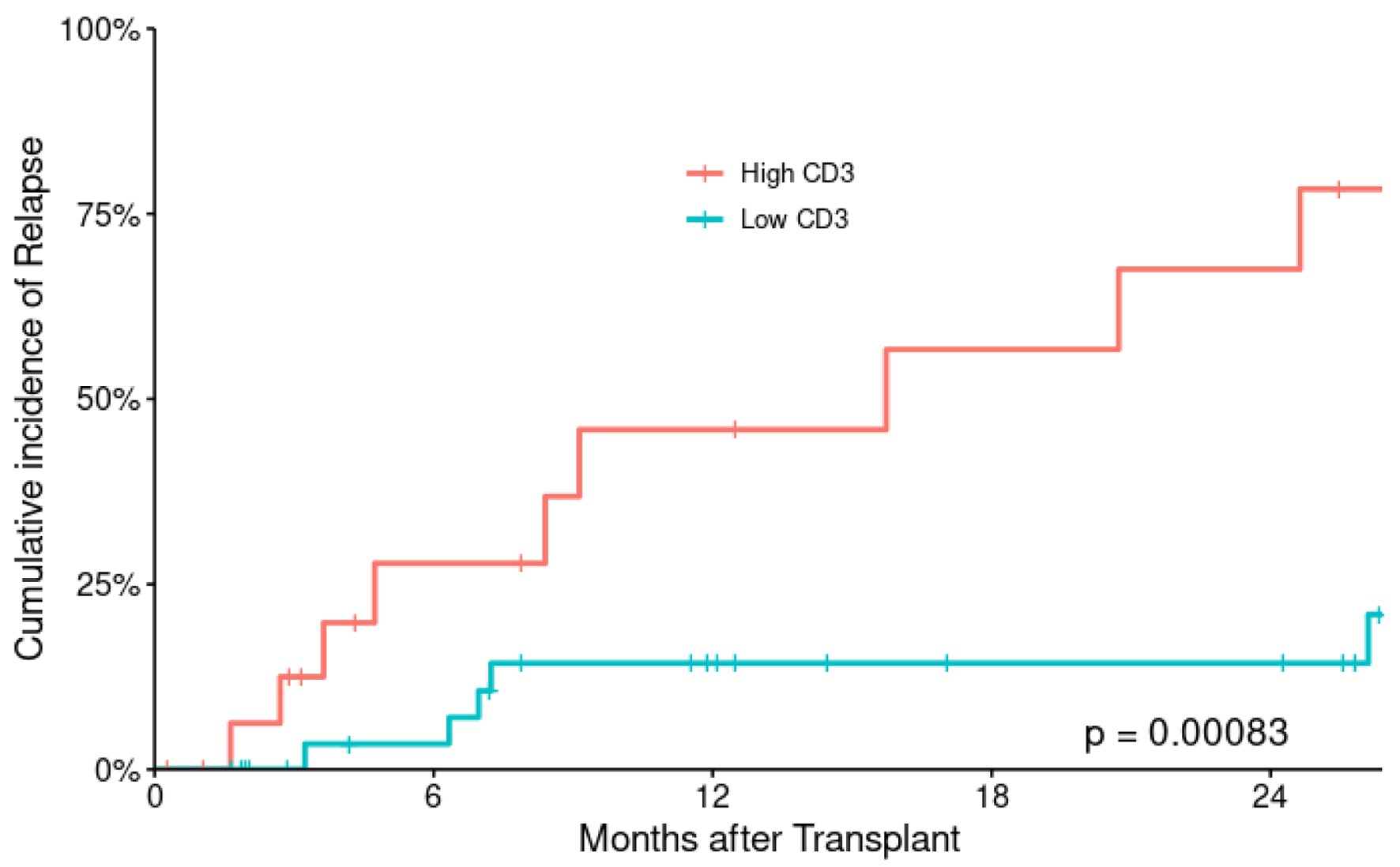
Figure 1. Cumulative incidence of relapse according to CD3+ T-cell dose.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 1, February 2023, pages 27-36
The Impact of Graft CD3+ T-Cell Dose on the Outcome of T-Cell Replete Human Leukocyte Antigen-Mismatched Allogeneic Hematopoietic Peripheral Blood Stem Cells Transplantation
Figures

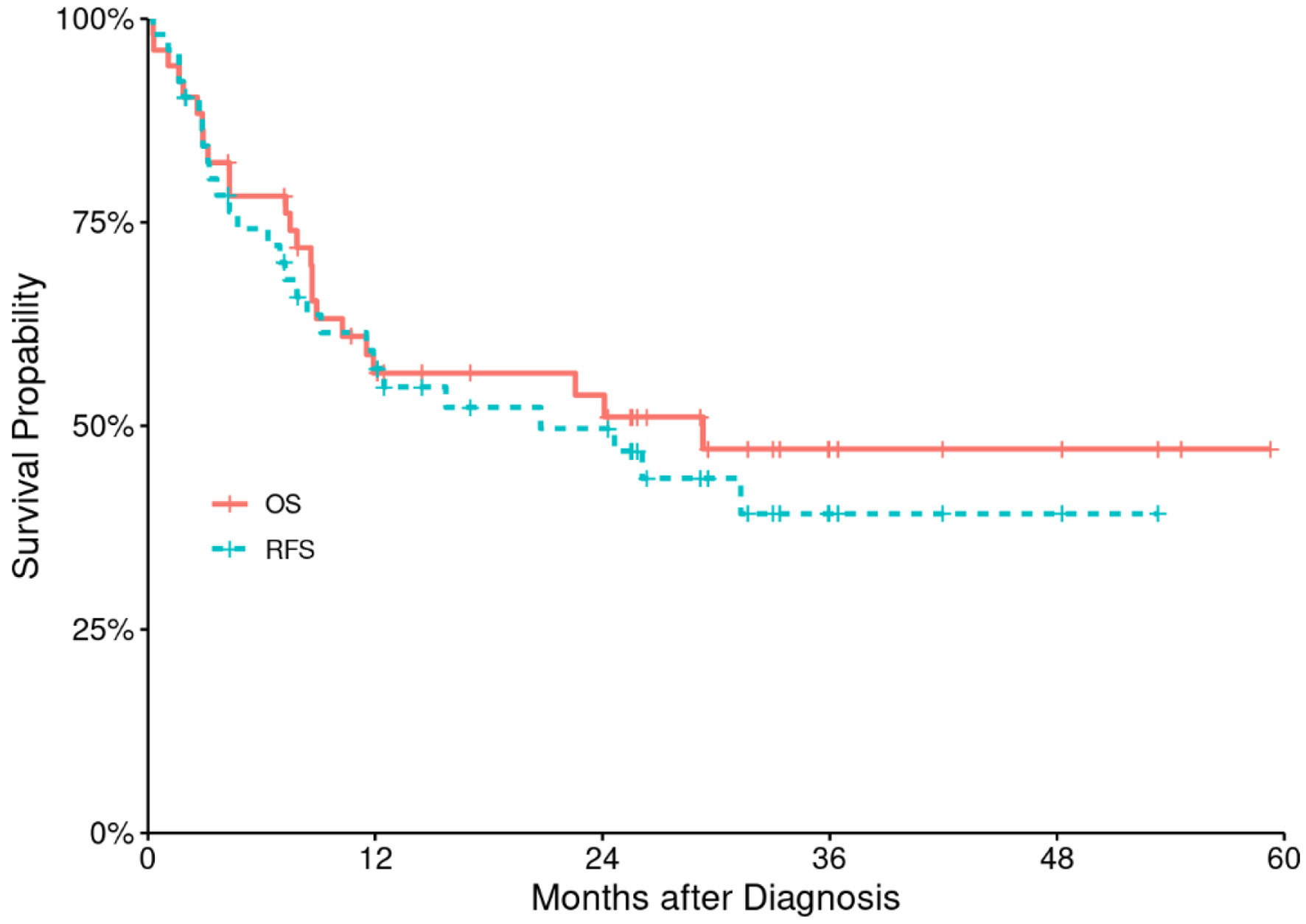
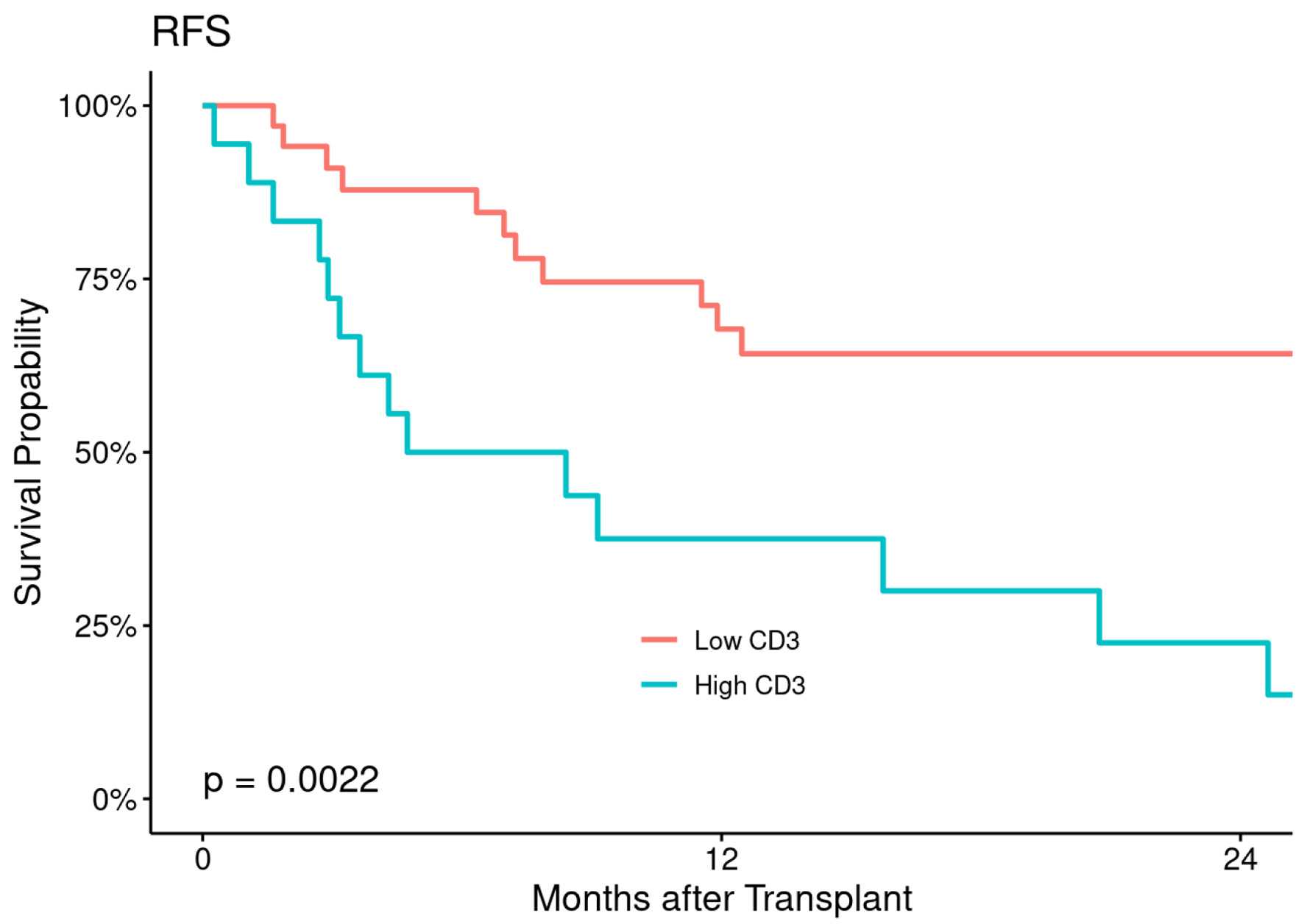
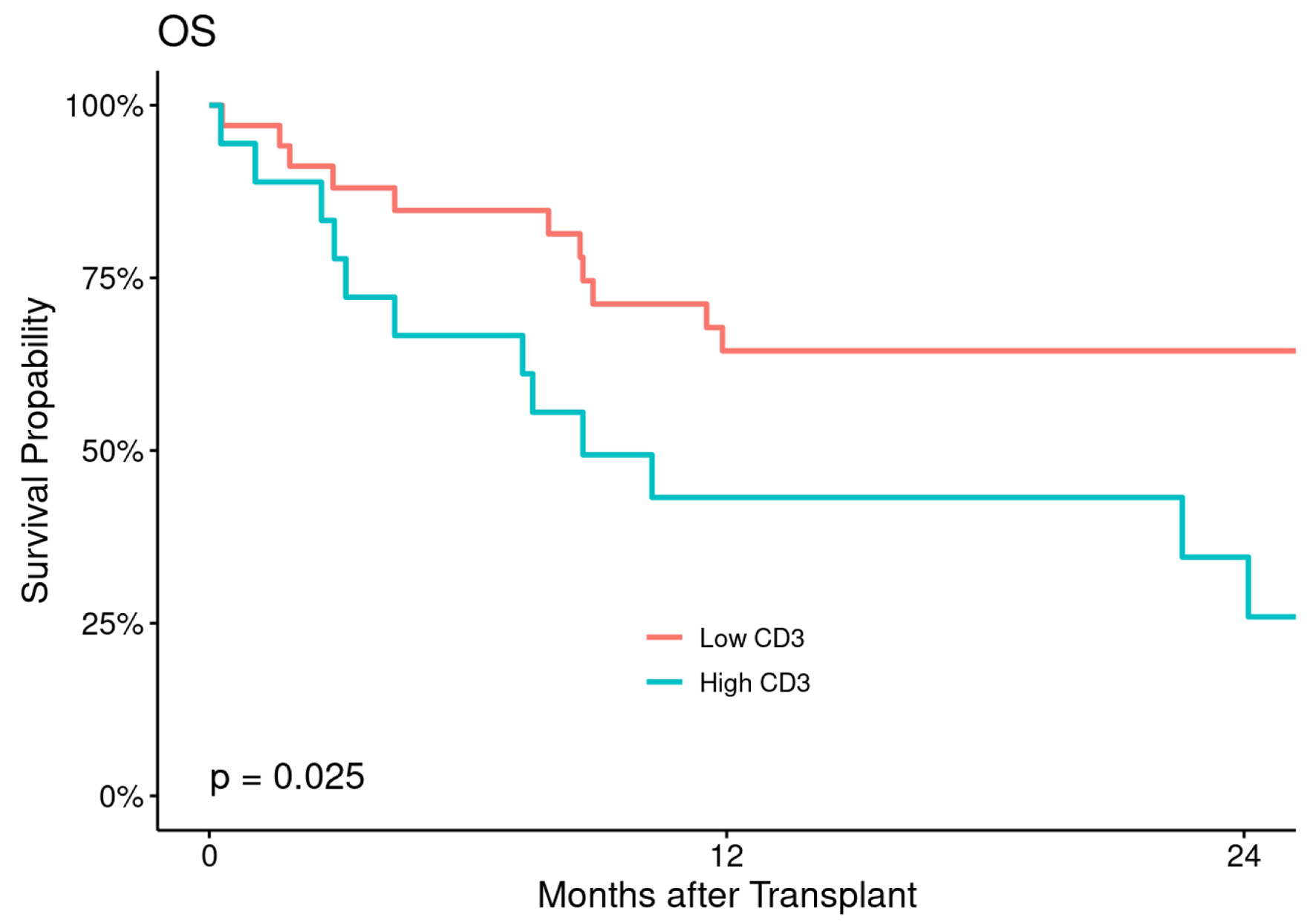
Table
| Variable | Low CD3+ T-cell dose (34 (65.4%)) | High CD3+ T-cell dose (18 (34.6%)) | P-value |
|---|---|---|---|
| R: recipient; D: donor; CMV: cytomegalovirus; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; ALL: acute lymphoblastic leukemia; HSCT-CI: hematopoietic stem cell comorbidity index; ATG: anti-thymocyte globulin; TBI: total body irradiation; GvHD: graft-versus-host disease; CSA: cyclosporine A; PTcy: post-transplant cyclophosphamide. TAC: Tacrolimus; MTX: methotrexate; NIH: National Institutes of Health. | |||
| Recipient age, median (range) | 29 (22 - 39) | 32 (25 - 40) | 0.6 |
| Donor age, median (range) | 28 (24 - 34) | 28 (21 - 42) | > 0.9 |
| Recipient gender | |||
| Female | 8 (24%) | 6 (33%) | 0.5 |
| Male | 26 (76%) | 12 (67%) | |
| Donor gender | |||
| Female | 9 (26%) | 12 (67%) | 0.4 |
| Male | 25 (74%) | 6 (33%) | |
| Recipient/donor sex match | |||
| Female/female | 4 (12%) | 4 (22%) | 0.021 |
| Male/female | 3 (8.8%) | 3 (17%) | |
| Donor/recipient ABO match | |||
| D-R | 17 (50%) | 8 (44%) | 0.7 |
| Recipient CMV seropositive | |||
| Negative positive | 1 (3.0%) | 0 (0%) | 0.6 |
| Positive-positive | 0 (0%) | 1 (5.6%) | |
| Positive-positive | 32 (97%) | 17 (94%) | |
| Nucleated cells, median (range) | 6.76 (5.72 - 7.84) | 7.78 (6.97 - 9.92) | 0.034 |
| CD3+ T-cell dose, median (range) | 17 (13 - 19) | 28 (25 - 30) | < 0.001 |
| CD34+ T-cell dose, median (range) | 7.0 (5.40 - 9.50) | 6.32 (5.03 - 8.54) | 0.40 |
| Disease type | |||
| ALL | 10 (29%) | 5 (28%) | 0.6 |
| AML-MDS | 24 (71%) | 13 (72.5%) | |
| HSCT-CI | |||
| 0-1 | 33 (97%) | 16 (89%) | 0.3 |
| 3-4 | 1 (2.9%) | 1 (5.6%) | |
| Pre-transplant disease status | |||
| Complete remission | 19 (61%) | 9 (56%) | 0.7 |
| Conditioning regimen | |||
| Myeloablative | 30 (88%) | 13 (72%) | 0.2 |
| Reduced intensity | 3 (8.8%) | 4 (22%) | |
| Reduced toxicity | 0 (0%) | 1 (5.6%) | |
| TBI-based conditioning | 14 (41%) | 10 (56%) | 0.3 |
| ATG | 6 (18%) | 3 (17%) | > 0.9 |
| Donor type | |||
| Haploidentical | 29 (85%) | 14 (78%) | 0.7 |
| Mismatched related | 5 (15%) | 4 (22%) | |
| GvHD prophylaxis | |||
| CSA or TAC + PTcy | 29 (85%) | 14 (78%) | 0.4 |
| CSA or TAC/MTX | 3 (8.8%) | 1 (5.6%) | |
| Acute GvHD | 17 (50%) | 7 (39%) | 0.4 |
| Chronic GvHD | |||
| Total | 10 (29%) | 4 (22%) | 0.7 |
| NIH score 2-3 | 6 (60%) | 3 (75%) | > 0.9 |