Figures
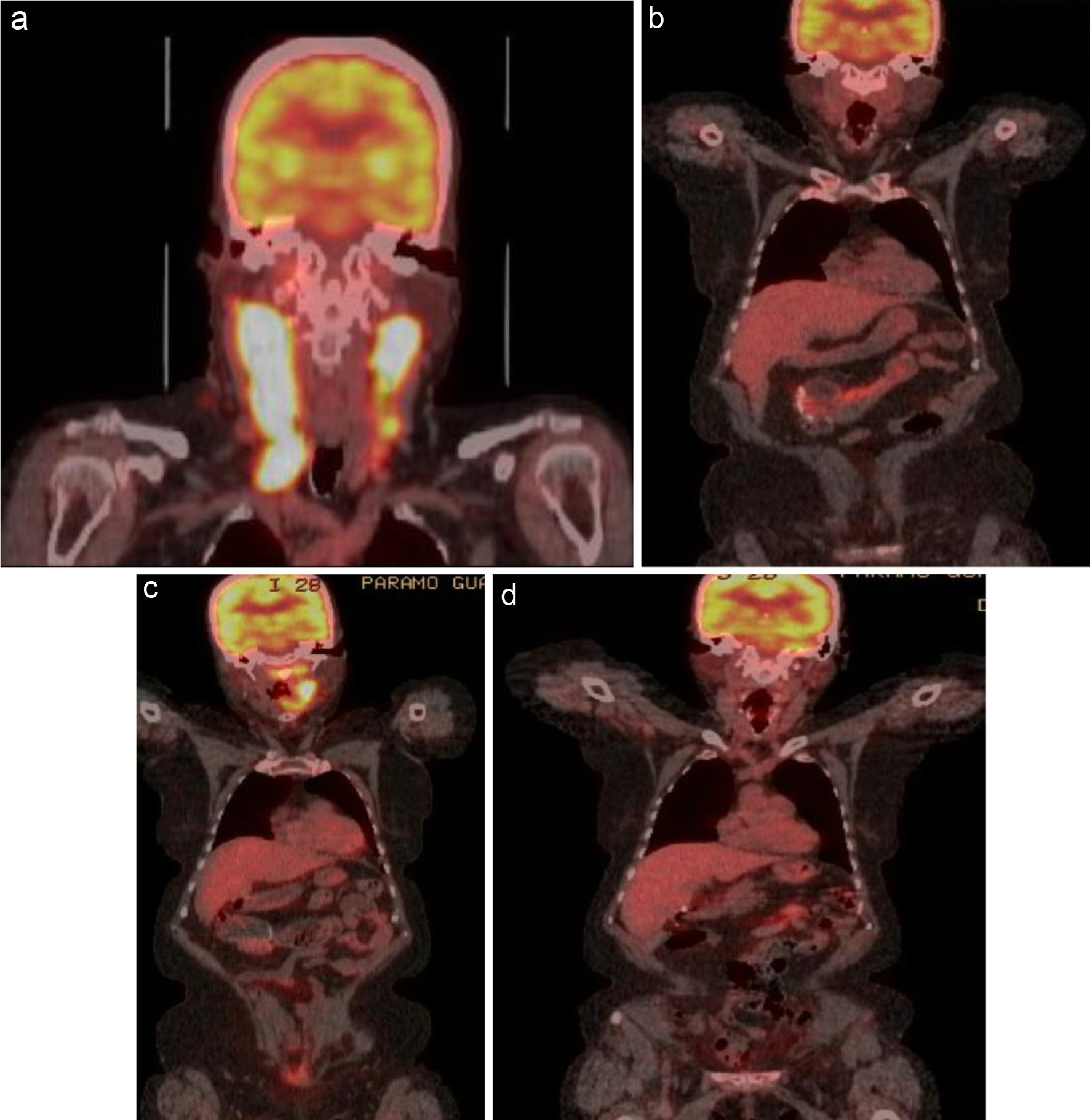
Figure 1. (a) FDG-avid uptake in the right oropharynx with an SUV of 27.4. Extensive lymph node involvement is present in the bilateral cervical chains (right greater than left), extending down to the level of the clavicle on the right. Areas of bilateral intensely FDG-avid lymph node conglomerates have an SUV of 27.4 on the right and 23 on the left. (b) PET/CT showing complete response after six cycles of R-CHOP. (c) PET/CT done 1 year later showing relapse. (d) Started on salvage therapy with R2-COP and achieved a CMR after two cycles. CMR: complete metabolic response; PET/CT: positron emission tomography/computed tomography; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; FDG: fluorodeoxyglucose; SUV: standardized uptake value; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide.
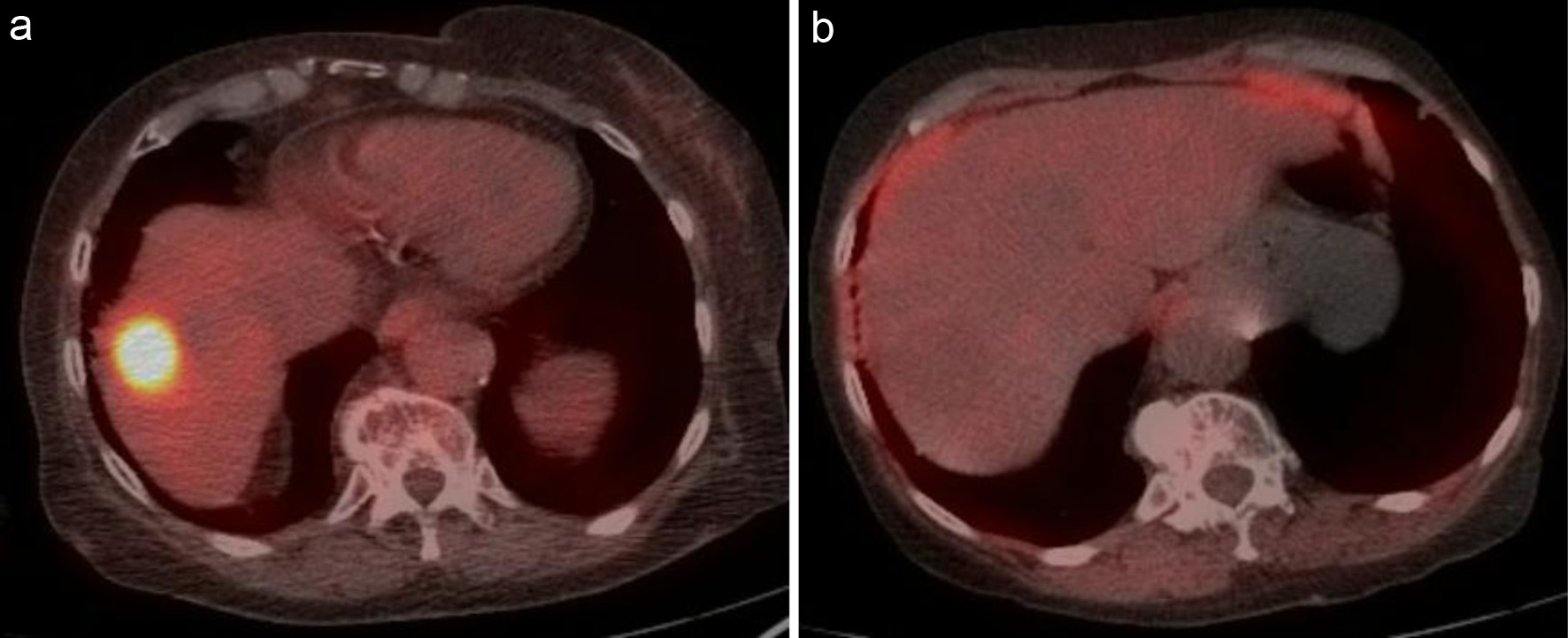
Figure 2. (a) Intense metabolically active lesion at the dome of the liver with an SUV of 21.7. (b) Interim PET/CT after two cycles showing complete resolution of abnormal metabolic activity within the liver lesion. PET/CT: positron emission tomography/computed tomography; SUV: standardized uptake value.
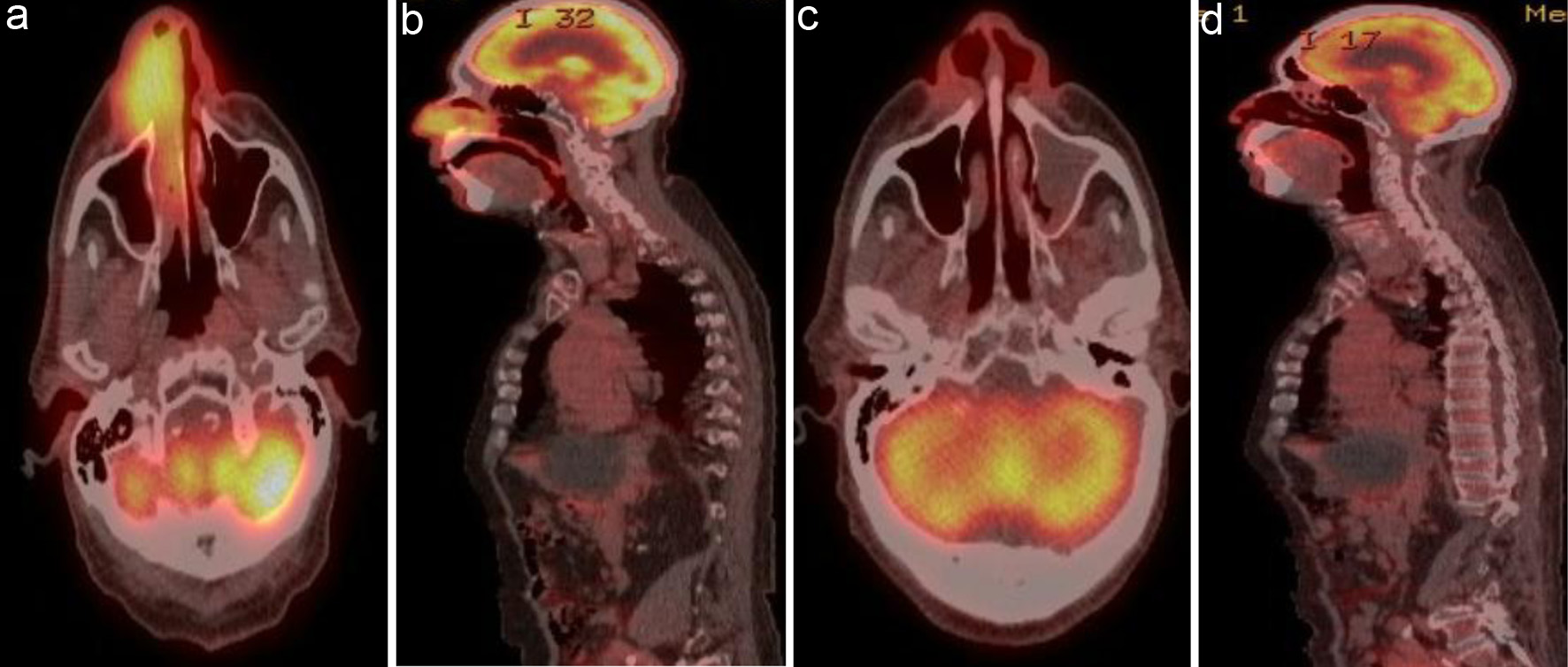
Figure 3. (a) Coronal view and (b) sagittal view of 5.1 × 3.6 cm hypermetabolic mass involving the right nasal ala with extension into the right nostril and nasal cavity. (c) Coronal view and (d) sagittal view of post-treatment PET/CT showing complete resolution of previously noted increased metabolic activity in the nasal cavity. PET/CT: positron emission tomography/computed tomography.
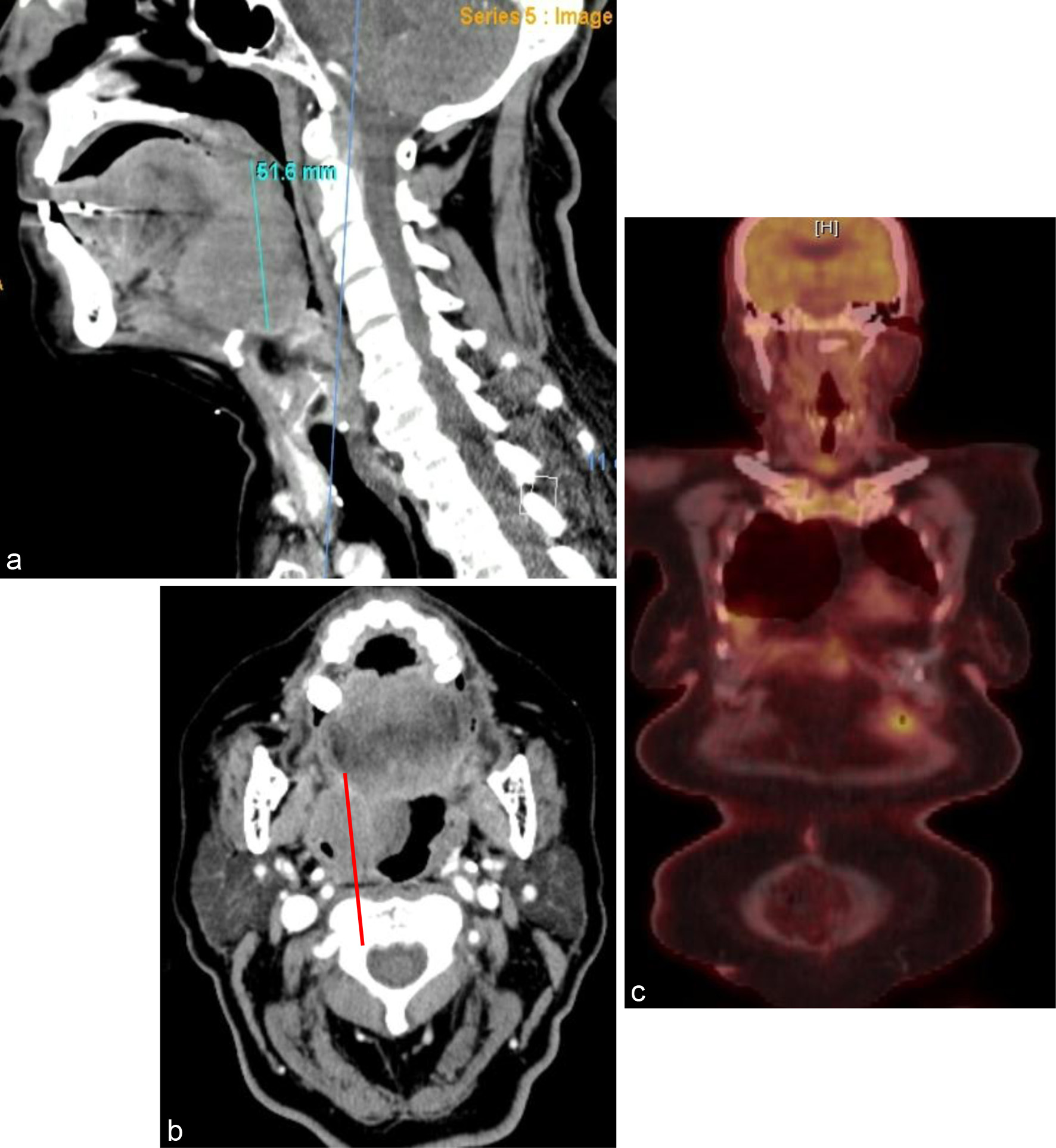
Figure 4. (a) Sagittal view and (b) coronal view of a 4.4 × 5.2 × 5.2 cm solid mass along the right base of the tongue with narrowing of the oropharynx. (c) Interim PET/CT after two cycles showing complete metabolic response. PET/CT: positron emission tomography/computed tomography.
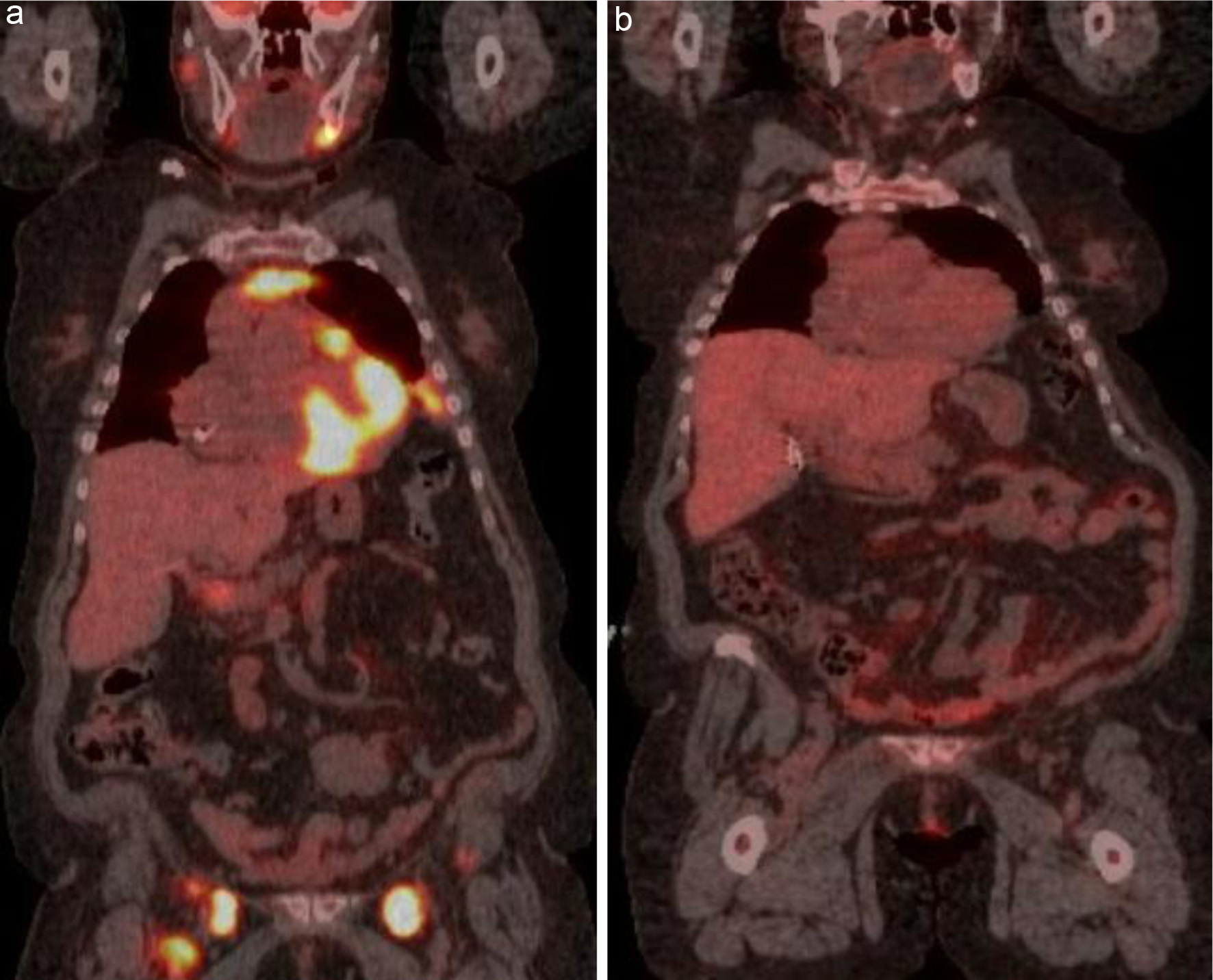
Figure 5. (a) FDG-avid lymphadenopathy in multiple stations above and below the diaphragm. (b) Interim PET/CT after three cycles showing a complete metabolic response. PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose.
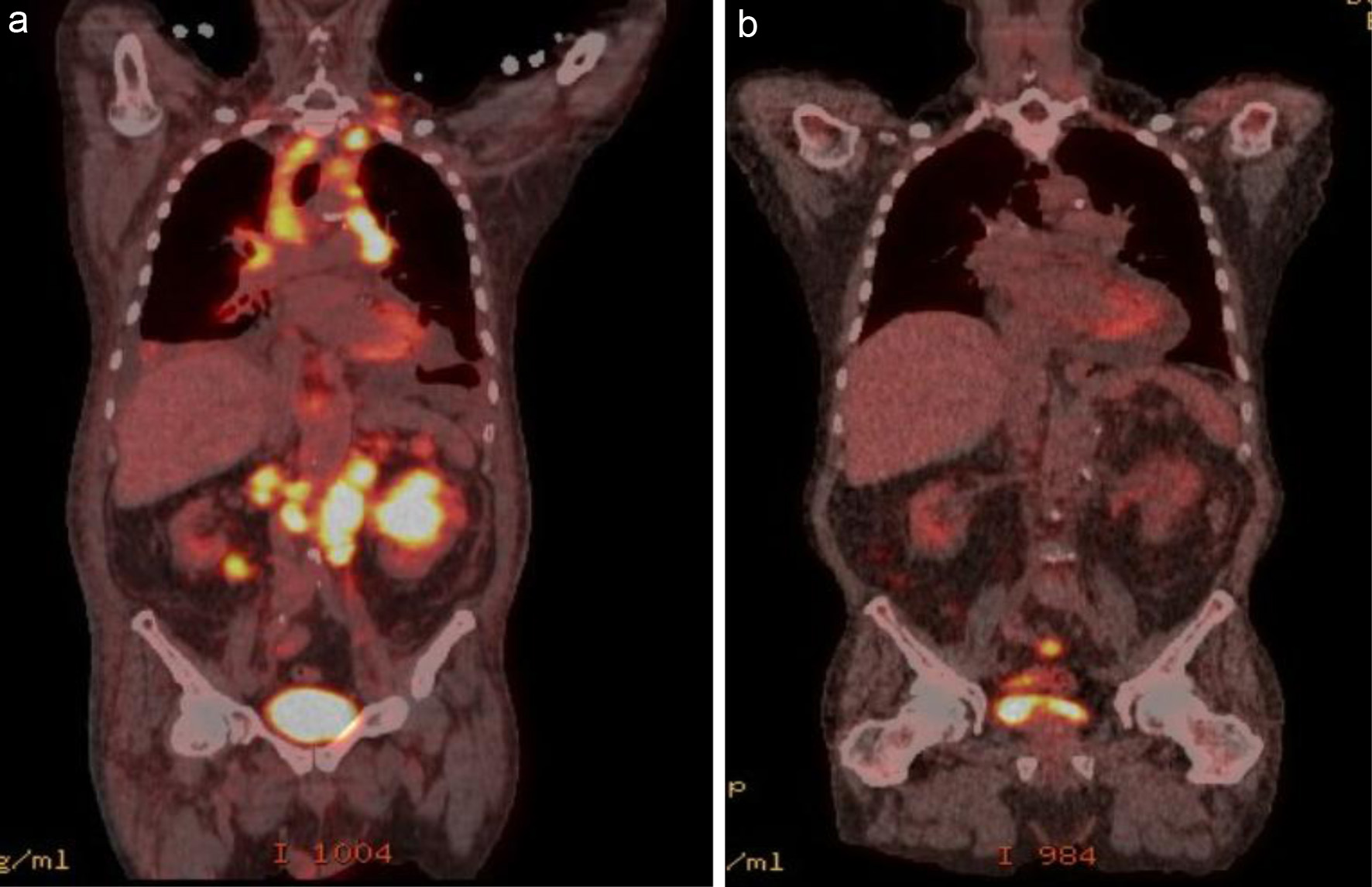
Figure 6. Pretreatment (a): extensive FDG-avid lymphomatous involvement above and below the diaphragm, including cervical, mediastinal, retrocrural, and retroperitoneal lymphadenopathy, as well as foci in the lungs, spleen, and bilateral kidneys. (b) PET/CT after four cycles of R2-COP showing a complete metabolic response. PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide.
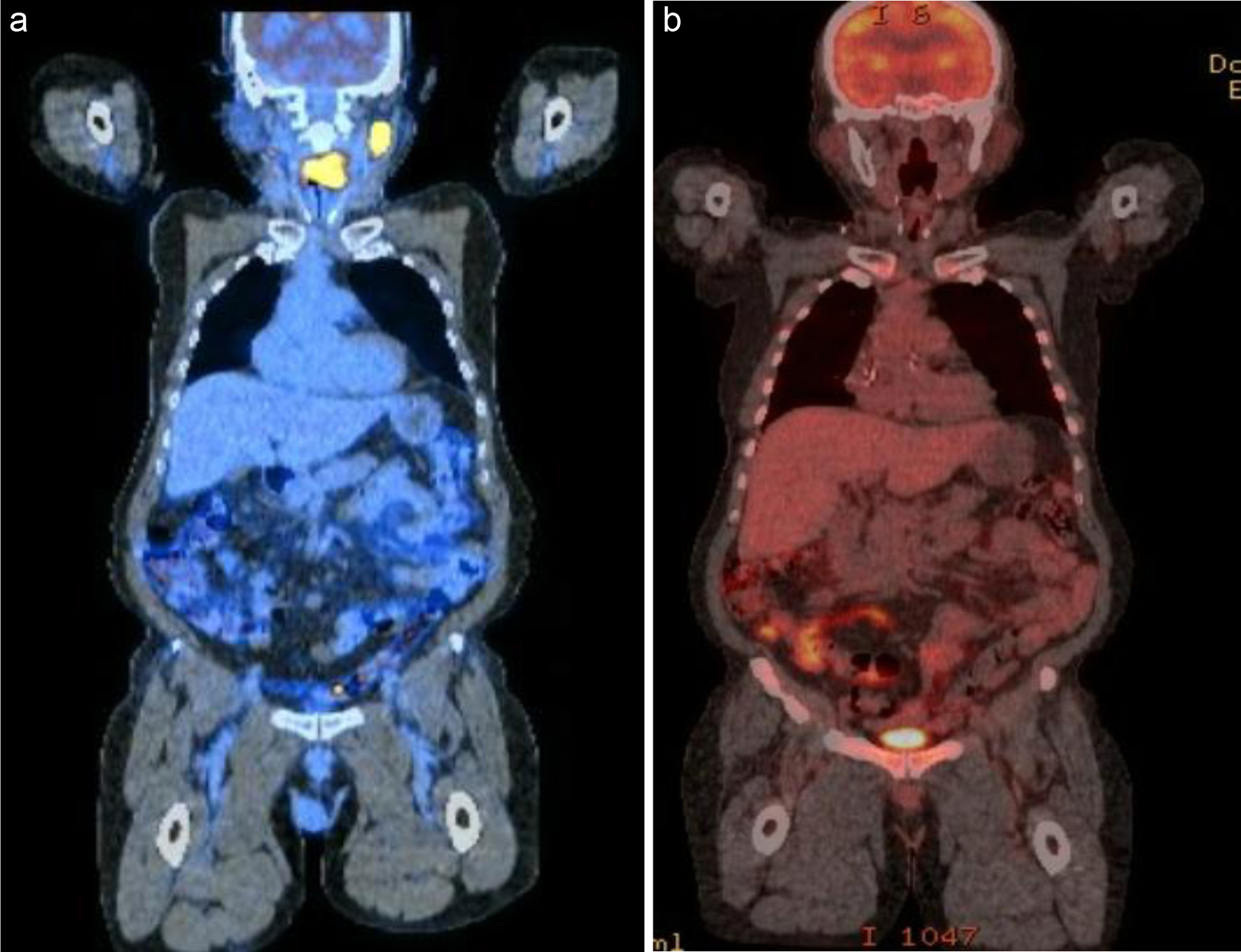
Figure 7. PET/CT scan showed a left tongue-base mass with intense FDG uptake, as well as lymphadenopathy in multiple levels of the left neck (a). He was treated with three cycles of R2-COP, and an interim PET scan showed a CMR (b). CMR: complete metabolic response; PET/CT: positron emission tomography/computed tomography; FDG: fluorodeoxyglucose; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide.
Tables
Table 1. Clinical Characteristics of Patients 1 - 7
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|
| ECOG: Eastern Cooperative Oncology Group; PS: performance status; DLBCL: diffuse large B-cell lymphoma; F: female; M: male; GCB: germinal center B-cell; ABC: activated B-cell; DEL: double expressor lymphoma; DHL: double hit lymphoma; THL: triple hit lymphoma; AJCC: the American Joint Committee on Cancer; LDH: lactate dehydrogenase; IPI: international prognostic index. |
| Age (years) | 76 | 83 | 79 | 74 | 72 | 87 | 72 |
| Sex | F | F | M | F | F | M | M |
| ECOG PS | 3 | 2 | 4 | 4 | 0 | 2 | 2 |
| Charlson comorbidity index | 5 | 12 | 6 | 6 | 7 | 6 | 6 |
| DLBCL subtype | GCB | GCB | GCB | ABC | GCB | ABC | ABC |
| Ki-67 | 60 | 90 | 90 | 95 | 80 | 95 | 80 |
| DEL/DHL/THL | No | No | No | DEL | No | DEL, FISH not available | No |
| AJCC stage | IVB | IVE | IE | IV | IV | IVB | IV |
| Albumin(g/dL) | 2.9 | 2.9 | 3.1 | 1.5 | 2.7 | 2.4 | 2.6 |
| LDH (U/L) | 154 | 227 | 213 | 270 | 256 | 627 | 328 |
| IPI score | 3 | 5 | 3 | 4 | 4 | 5 | 4 |
| Extranodal sites | Tonsil, nasopharynx | Liver | Left nostril | Oropharynx | Lung | Lungs, spleen, and bilateral kidneys | Oropharynx |
| Bone marrow involvement | No | No | No | No | No | No | No |
Table 2. Treatment and Response in Patients 1 - 7
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|
| aRelapsed 1 year later. bDue to lenalidomide intolerance, treatment changed to cyclophosphamide, prednisone, and rituximab only for the last three cycles. Dosing: rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, oral prednisone 100 mg daily for 5 days, and vincristine 1 mg. CMR: complete metabolic response; CR: complete response; DLBCL: diffuse large B-cell lymphoma; PET/CT: positron emission tomography/computed tomography; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CP: rituximab, cyclophosphamide, and prednisone; R2-COP: R-COP (rituximab, cyclophosphamide, vincristine, and prednisone) in combination with lenalidomide. |
| Prior treatment, response | 6 cycles R-CHOP, achieved CR (2018)a | Newly diagnosed DLBCL (patient undergoing hormonal therapy for stage 4 breast cancer) | None | 1 cycle R-CHOP (2018) | None | None | None |
| Latest treatment | 6 cycles R-CP + lenalidomide | 3 cycles R2-COP (achieved CR)b | 6 cycles R2-COP | 5 cycles R2-COP | 6 cycles R2-COP | 6 cycles R2-COP | 6 cycles R2-COP |
| Lenalidomide dose | 25 mg × 21 days | 25 mg × 21 days | 25 mg × 21 days | 10 mg × 14 days | 25 mg × 14 days | 25 mg × 14 days | 20 mg × 21 days |
| Interim PET/CT scan, result | After 2 cycles, CMR | After 3 cycles, CMR | NA | After 2 cycles, CMR | After 3 cycles, CMR | After 4 cycles, CMR | After 3 cycles, CMR |
| Treatment duration, months | 6 | 6 | 6 | 5 | 6 | 6 | 6 |
| Best response | CR | CR | CR | CR | CR | CR | CR |
| Follow-up, months | 24 | 44 | 4 | 24 | 20 | 48 | 3 |
| Major treatment related-adverse event | No cytopenias | Grade 3 neutropenia | Grade 2 thrombocytopenia | Grade 4 neutropenia managed with growth factors | No cytopenias | Grade 3 neutropenia, one hospitalization for infection | Hepatitis B reactivation |
| Outcome | Alive, remains in CR | Alive, lymphoma remains in CR | Alive, remains in CR | Alive, remains in CR | Alive, remains in CR | Alive, remains in CR | Died from hepatitis B reactivation |






