Baena et al
Colombia | Retrospective
2013 - 2019 | MSD: 18
HRD: 32 | Neu: 94% median 17 days
Plt: 94% median 18 days | MSD (II-IV): 44%
HRD (II-IV): 43% | MSD: 50%
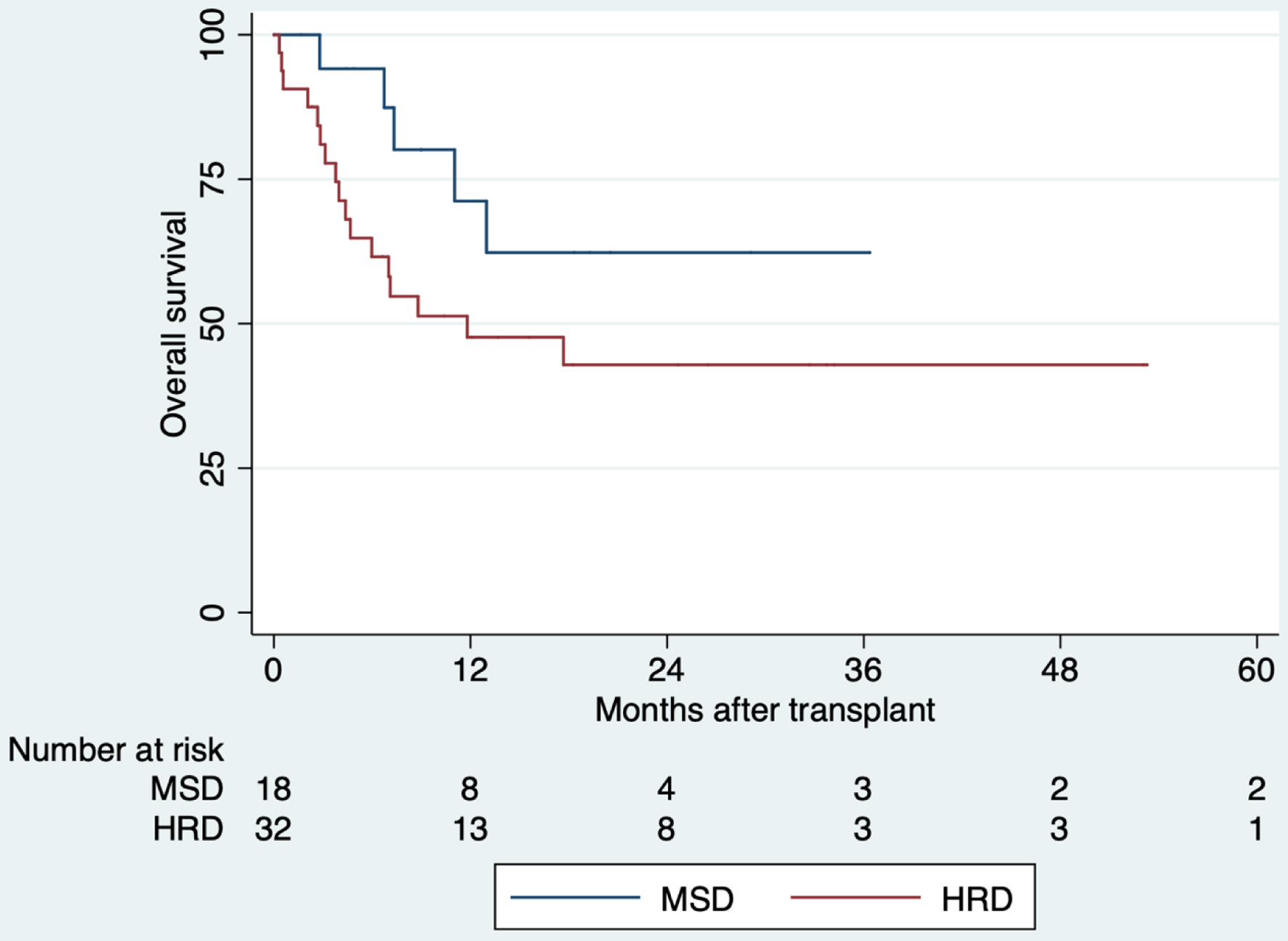
HRD: 40% | MSD: 62% 5 years
HRD: 43% 5 years | MSD: 77% 5 years
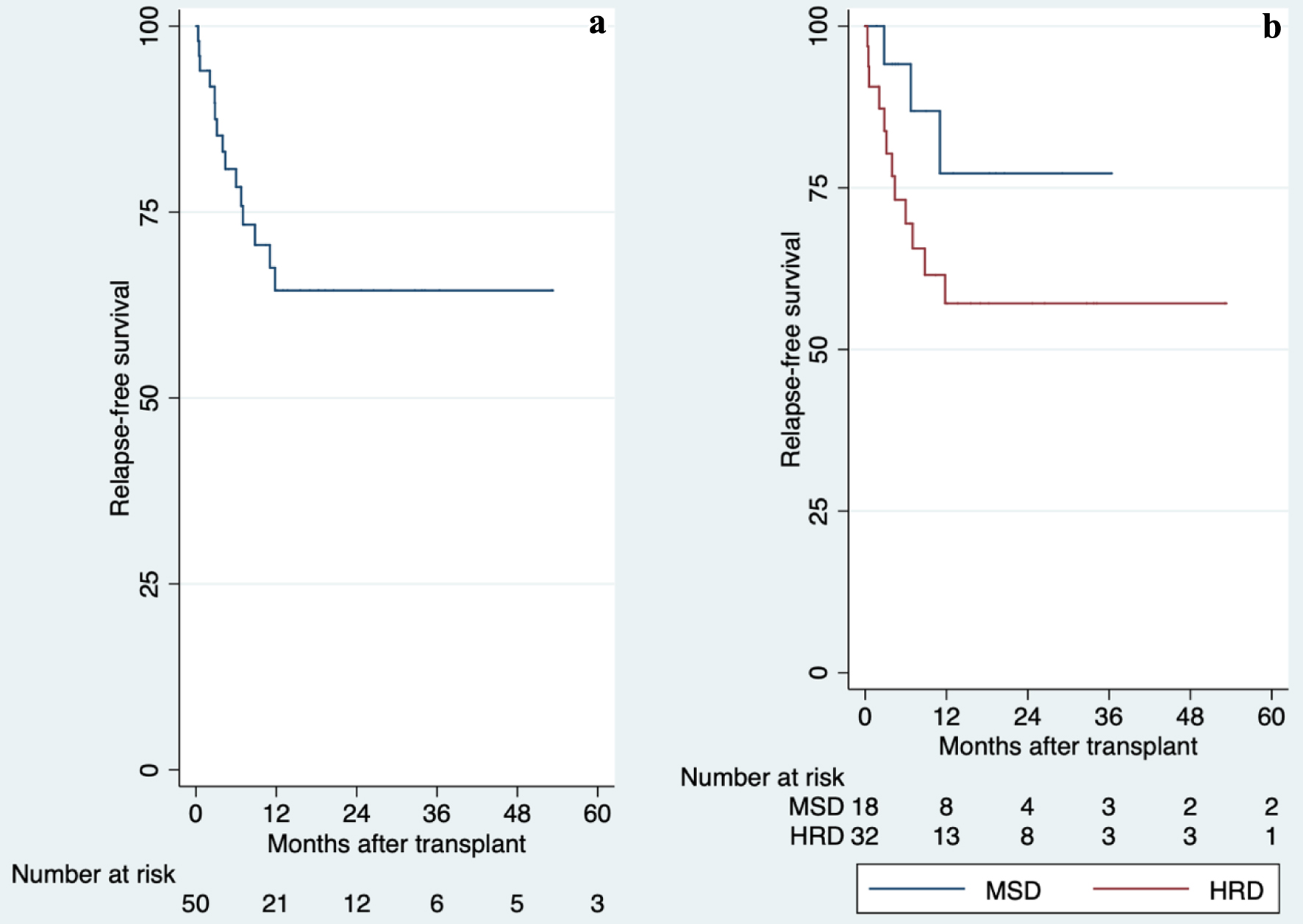
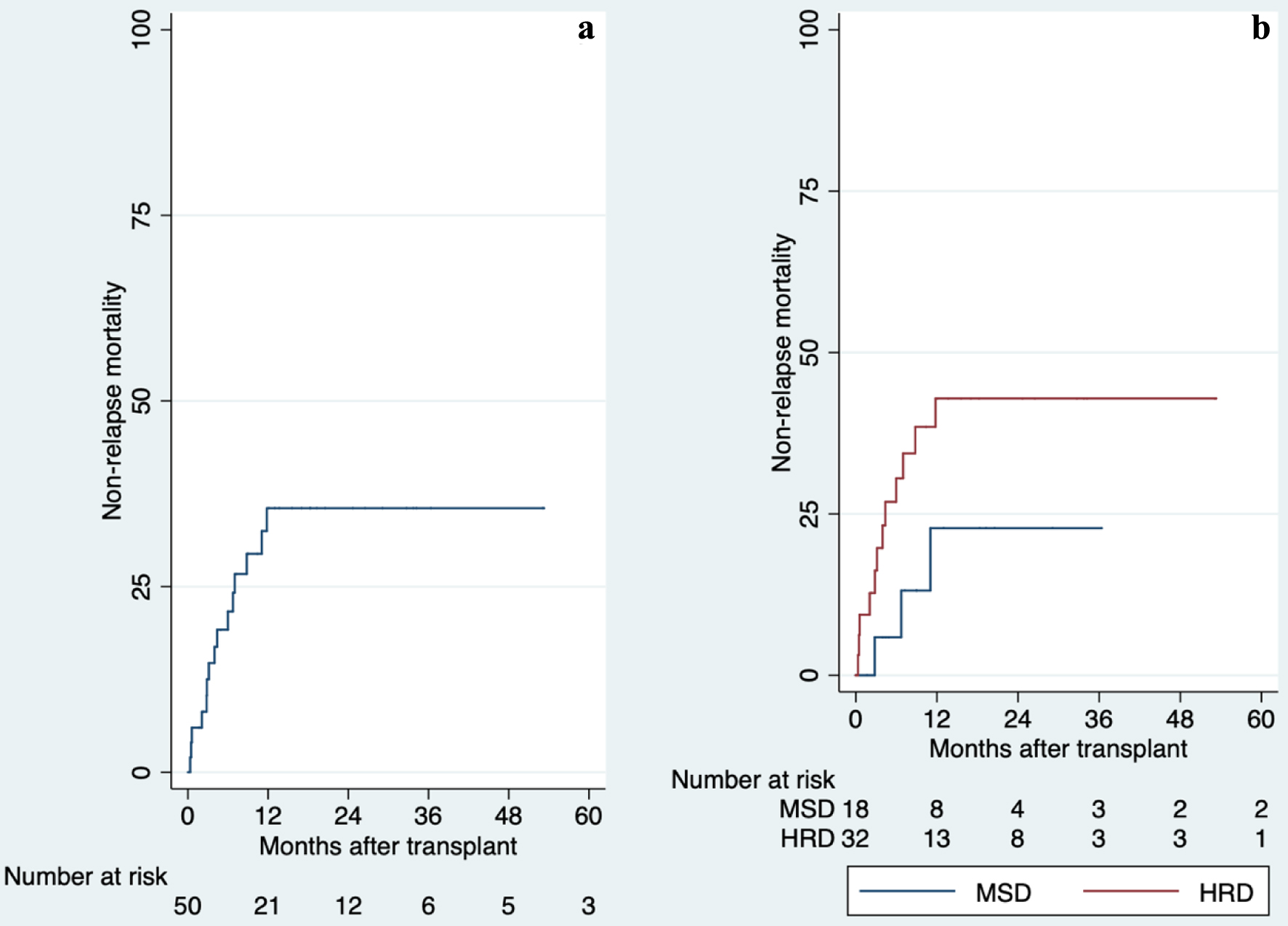
HRD: 57% 5 years | MAC with TBI: 100% | MSD: 6% day-100
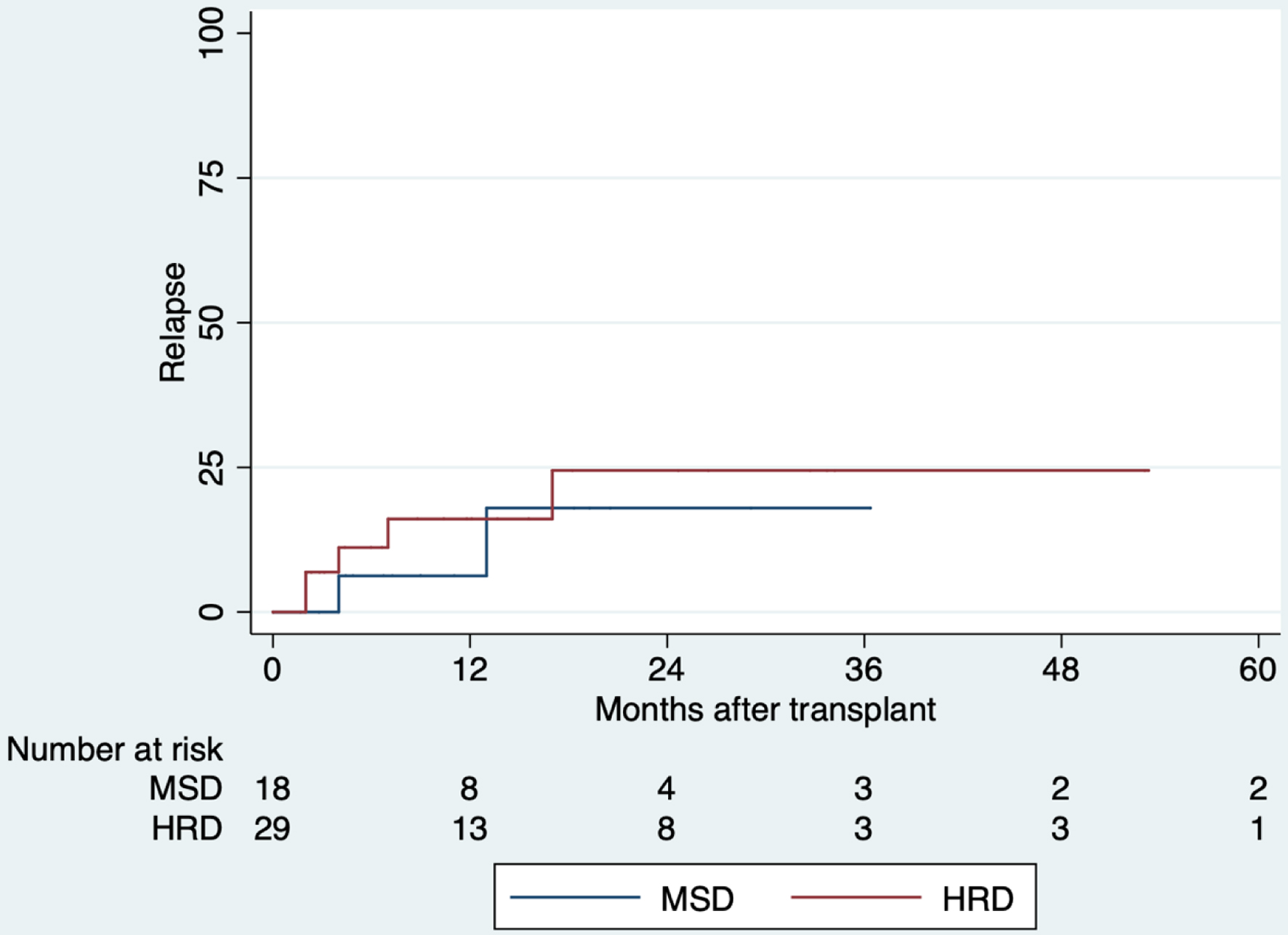
HRD: 20% day-100 | MSD: 18% 5 years
HRD: 25% 5 years |
Abello et al, 2008 [20]
Colombia | Retrospective
1993 - 2007 | 46 | - | - | - | 53.2%a | 50.9%a | MAC | - | - |
Leon Rodriguez et al, 2017 [21]
Mexico | Retrospective
1998 - 2017 | 19 (15%) | Neu: median 20 days
Plt: median 16 days | 21%a | 33%a | 48% 5 years | 65% 10 yearsa | MAC: 75%
NMA: 25% | 36%a | 58%b |
Trucco et al, 2017 [22]
Argentina | Retrospective
2012 - 2016 | MRD: 89 (36%)
HRD: 83 (33%) | MRD: 35% ≥ 15 days
HRD: 85% ≥ 15 days | MRD (II-IV): 27%
HRD (II-IV): 33% | MRD: 22%
HRD: 25% | MRD: 51% 3 years
HRD: 34% 3 years | MRD: 42% 3 years
HRD: 31% 3 years | MAC: 81% | MRD: 15% 3 years
HRD: 29% 3 years | MRD: 44% 3 years
HRD: 40% 3 years |
Versluis et al, 2017 [23]
Europe | Retrospective
2000 - 2014 | MRD: 3,511
HRD: 193 | MRD: 16 days
HRD: 18 days | MRD (II-IV): 22%
HRD (II-IV): 25% | MRD: 38±1% 2 years
HRD: 37±4% 2 years | MRD: 59±1% 2 years
HRD: 57±4% 2 years | MRD: 53±1% 2 years
HRD: 52±4% 2 years | MRD: MAC 56%
HRD: MAC 54% | MRD: 15±1% 2 years
HRD: 26±3% 2 years | MRD: 30% 2 years
HRD: 22±3% 2 years |
Ostgard et al, 2018 [24]
Denmark | Cohort
2000 - 2014 | HRD: 196
MRD: 91 (46.4%) | - | - | - | Median 1,173 days | Median 1,068 days | MAC
Cy TBI 90%
BU-Cy 9%
FLU-Treosulfan 1% | - | 24% |
Salvatore et al, 2018 [25]
Europe | Retrospective
2007 - 2015 | HRD: 185
MRD: 2,469 | | HRD (II-IV): 36%
MRD (II-IV): 24% | HRD: 39%
MRD: 33% | HRD: 67%
MRD: 66% | - | MAC: HRD 50%, MRD 53%
RIC: HRD 50%, MSD 47% | HRD: 18% 2 years
MRD: 10% 2 years | HRD: 21%
MRD: 36% |
Liu et al, 2019 [26]
China | Retrospective
2008 - 2015 | MSD: 43
HRD: 127 | Neu: 100% median 14.9 days
Plt: 94.1%
median 20.7 days | General (II-IV/III-IV): 40%/12.9%
MRD (II-IV/III-IV): 27.9%/7%
HRD (II-IV/III-IV): 44.1%/15% | - | General: 63.9% 3 years
MRD: 63.0%
HRD: 64.1% | General: 59.7%
MRD: 51.8%
HRD: 62.7% | HRD: cytarabine, BU, Cy, simustine, thymoglobulin
MRD: hydroxyurea, cytarabine, BU, Cy, simustine | - | - |
Rashidi et al, 2019 [27]
USA | Retrospective
2008 - 2015 | MRD: 869
HRD: 336 | MRD: Neu 99% 1 month
Plt 97% 100 days
HRD: Neu: 92% 1 month
Plt 89% 100 days | MRD (II-IV): 30% 6 months
HRD (II-IV): 32% 6 months | MRD: 56% 3 years
HRD: 26% 3 years | MRD: 55% 3 years
HRD: 48% 3 years | MRD: 3 years 48%, HRD: 3 years 43% | MRD: MAC without TBI 45%, MAC with TBI 24%
HRD: RIC/NMA 65%, MAC without TBI 25% | MRD: 14% 3 years HRD: 19% 3 years | MRD: 38% 3 years
HRD: 38% 3 years |
Figueroa et al, 2020 [28]
Colombia | Descriptive cohort
2015 - 2018 | 66 | - | MRD (III-IV): 23.7%
HRD (III-IV): 12.8%b | - | MRD: 72% 2 years
HRD: 42% 2 yearsb | - | MAC | - | MRD: 25%
HRD: 22%b |
Yanada et al, 2020 [29]
Japan | Retrospective
1992 - 2016 | 5,638 | Neu: 14 days
Plt: 21 days | 35% 100 days | 39% 2 years | 41% 5 years | - | MAC: 71%
RIC: 29% | 26% 5 years | 36% 5 years |
Mehta et al, 2022 [30]
USA | Retrospective
2015 - 2020 | HRD: 661
MRD: 140 | HRD: Neu 19 days, Plt 27 days
MRD: 15 days, Plt 22 days | HRD (II-IV): 44% 180 days
MRD (II-IV): 44% 180 days | HRD: 15%
MRD: 16% | HRD: 50%
MRD: 71% | - | MAC: HRD 24%, MRD 74%
RIC: HRD 76%, MRD 26% | HRD: 32%
MRD: 11% | HRD: 25%
MRD: 30% |
Rieger et al, 2023 [31]
Switzerland | Retrospective
2015 - 2020 | MSD: 68
HRD: 40 | HRD: Neu median 16 days, Plt 26.5 days
MRD: Neu median 12 days, Plt 15 days | MRD (II-IV): 14.7%
HRD (II-IV): 27.5% | - | HRD: 50% 2 years
MRD: 77% 2 years | - | MAC
BU-Cy-ATG: MRD 30.9%, HRD 0%
BU-Cy: MRD 2.9%, HRD: 22.5%
FLU-Cy-sTBI: MRD 0%, HRD: 12.5%
Other: MRD 7.4%, HRD 0%
RIC regimens
FLU-BU-ATG: MRD 48.5%, HRD 0%
FLU-BU: MRD 2.9%, HRD 65%
Other: MRD 7.4%, HRD 0% | HRD: 18% 2 years
MRD: 2.9% 2 years | - |