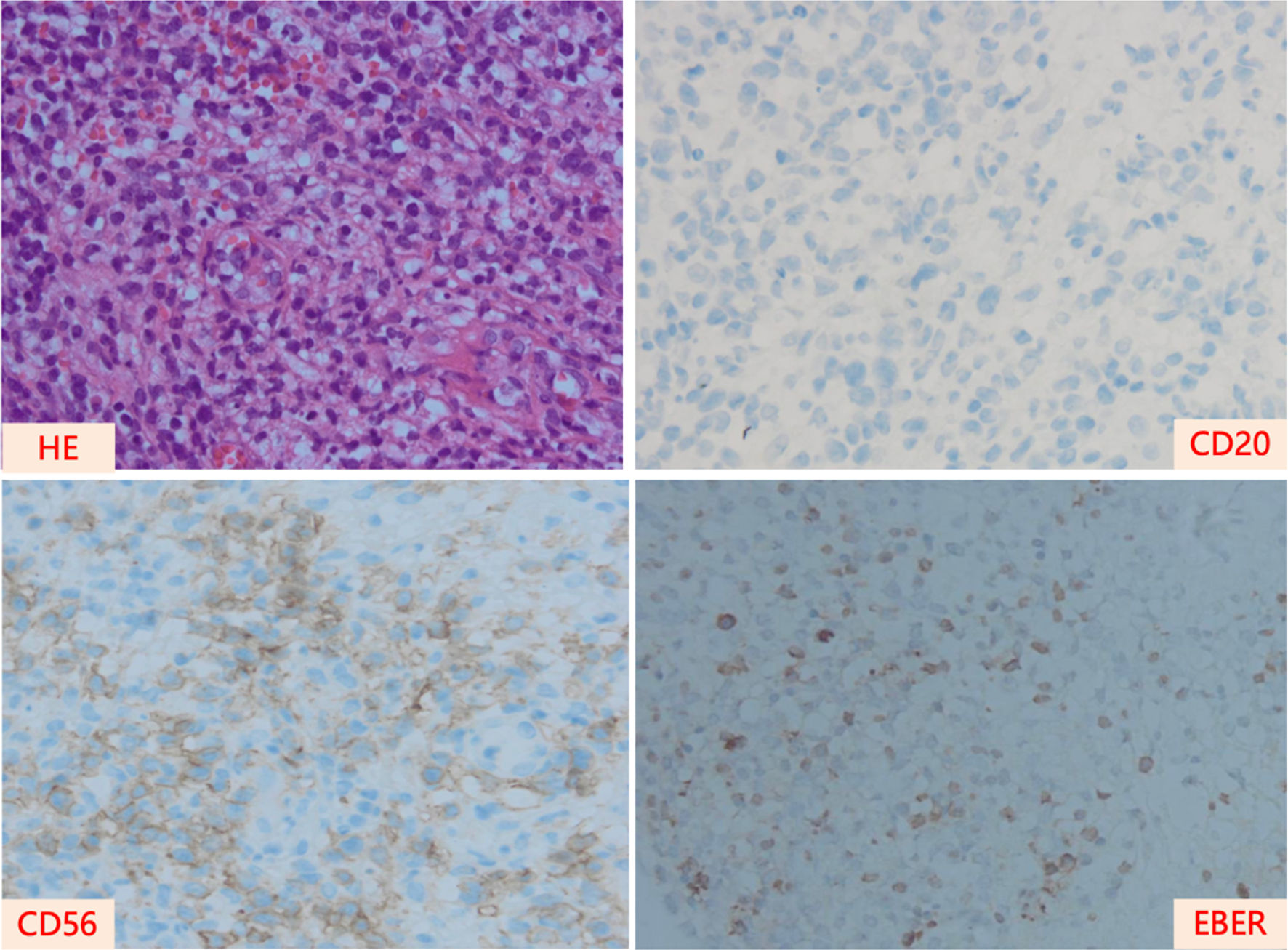
Figure 1. Pathological images of the nasal cavity of the patient at the time of diagnosis. HE: hematoxylin and eosin stain; EBER: EBV-encoded RNA.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 1-2, April 2024, pages 46-51
Clinical Efficacy of Programmed Cell Death Ligand 1 Antibody in Treatment of Extranodal Natural Killer/T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis
Figures


Table
| Reference | Targets | Secondary cause | Efficacy |
|---|---|---|---|
| sHLH: secondary hemophagocytic lymphohistiocytosis; IL-1R: interleukin-1 receptor; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; JAK: Janus kinase; OS: overall survival; DoR: duration of response; CRR: complete response rate; EBV: Epstein-Barr virus; MDS: myelodysplastic syndrome; ENKTCL-LAHS: extranodal natural killer/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis; ENKTCL: extranodal natural killer/T-cell lymphoma. | |||
| Miettunen et al, 2011 [17] | IL-1R | Rheumatic diseases | OS 2 - 40 months |
| Keith et al, 2012 [18] | CD52 | Systemic lupus erythematosus | DoR 19 months |
| Liu et al, 2020 [16] | PD-1 | EBV-associated HLH | OS 5 - 18.9 months |
| He et al, 2023 [19] | PD-1 + chemotherapy | ENKTCL-LAHS | OS 5.2 months |
| Song et al, 2023 [20] | JAK1/2 | Rheumatic diseases/lymphoma/pregnancy/MDS | ORR 50% |
| Dufranc et al, 2020 [21] | IL-6R | Infection/ autoimmune disorders | CRR 88.9% |
| Present case | PD-L1 | ENKTCL | DoR 44 months |