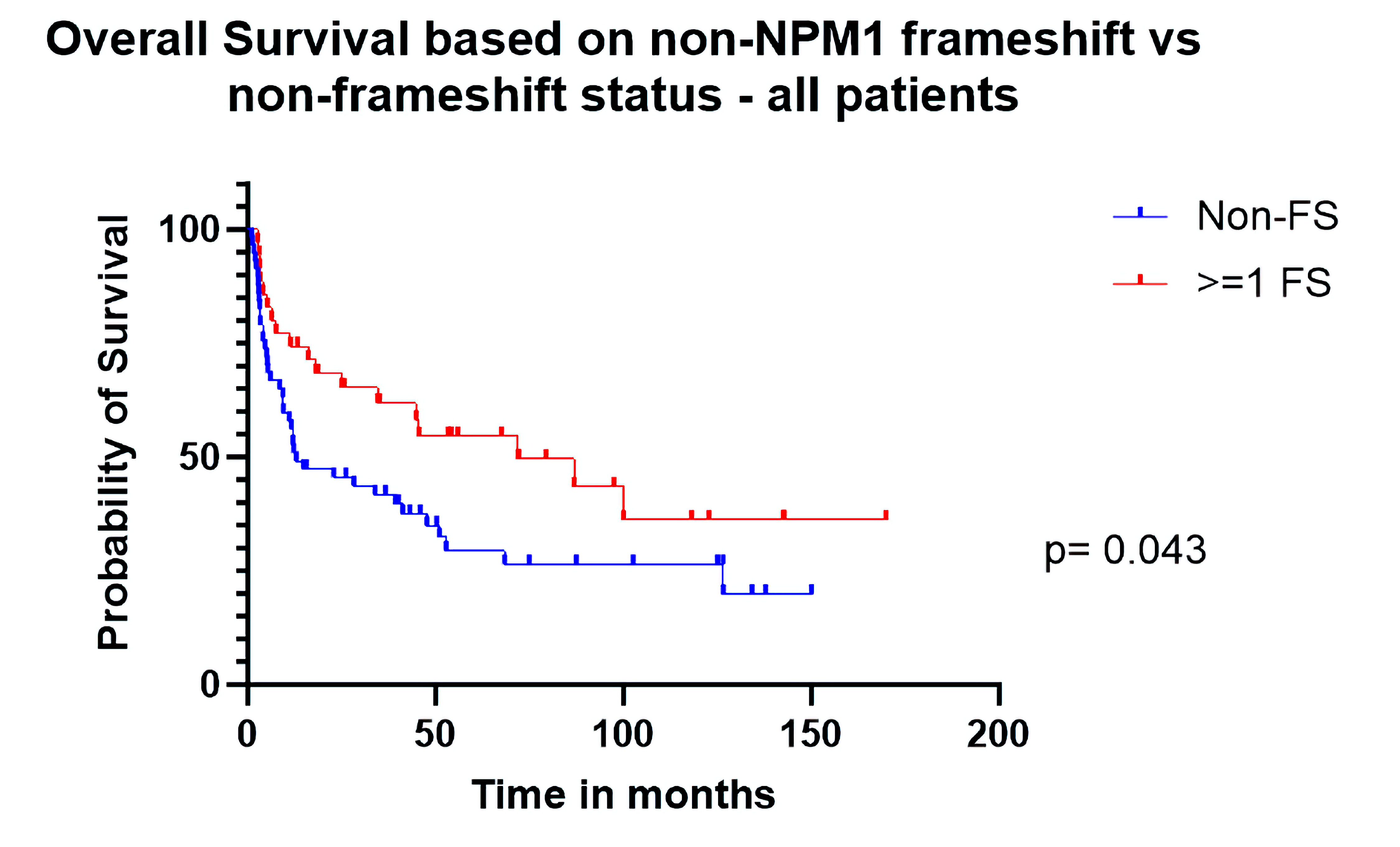
Figure 1. Overall survival based on non-NPM1 frameshift (FS) vs. non-FS status (all patients).
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 13, Number 3, June 2024, pages 86-93
Frameshift Mutations in Leukemia-Associated Genes Correlate With Superior Outcomes in Patients Undergoing Allogeneic Stem Cell Transplant for De Novo Acute Myeloid Leukemia
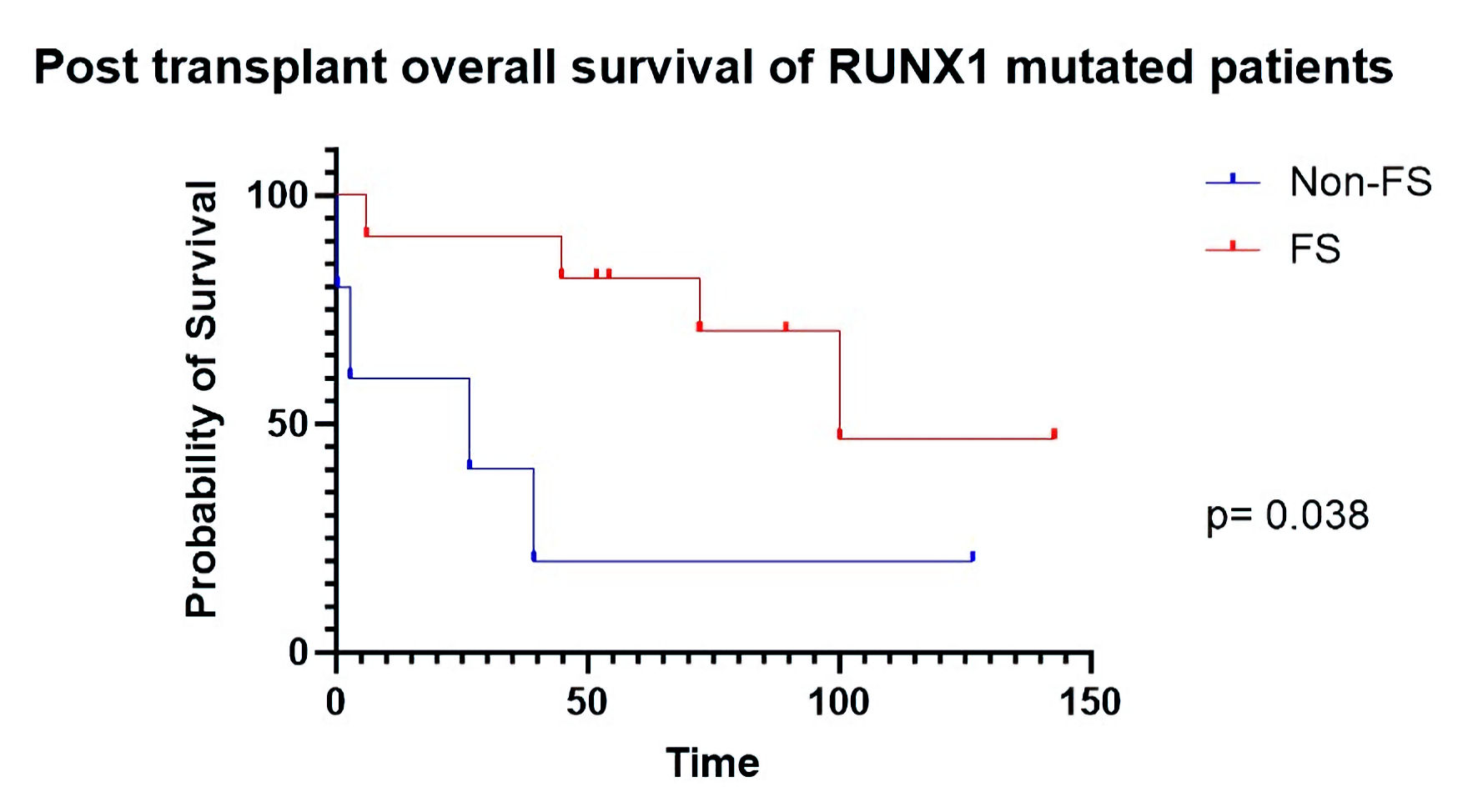
Figures

Tables
| Variables | No frameshift | ≥ 1 non-NPM1 frameshift | P value | |
|---|---|---|---|---|
| *P < 0.05. NCCN: National Comprehensive Cancer Network; IQR: interquartile range; MUD: matched unrelated donor; MSD: matched sibling donor; IV: intravenous; GvHD: graft-vs-host disease; AML: acute myeloid leukemia; Ara-C: cytarabine; HiDAC: high-dose cytarabine. | ||||
| Age (median) | 62 (IQR: 49 - 69) | 65 (IQR: 55 - 72) | 0.57 | |
| Disease risk category (NCCN) | 0.011* | |||
| Favorable; n (%) | 11 (11) | 9 (16) | 2 (5) | |
| Intermediate | 29 (31) | 22 (38) | 7 (18) | |
| Adverse | 55 (58) | 26 (45) | 29 (76) | |
| Transplant type | 0.17 | |||
| MUD | 27 (63) | 15 (48) | 12(75) | |
| MSD | 8 (19) | 7 (23) | 1 (11) | |
| Cord | 8 (19) | 5 (16) | 3 (19) | |
| Haploidentical | 4 | 4 (13) | 0 (0) | |
| Missing | 48 | 26 | 22 | |
| Conditioning regimen | 0.61 | |||
| High-dose mitoxantrone and Ara-C | 16 (66) | 9 (56) | 7 (88) | |
| IV arsenic | 1 (4) | 1 (6) | 0 (0) | |
| Cyclophosphamide and etoposide | 1 (4) | 1 (6) | 0 (0) | |
| Clofarabine | 1 (4) | 1 (6) | 0 (0) | |
| HiDAC | 5 (21) | 4 (25) | 1 (12) | |
| Missing | 71 | 41 | 29 | |
| Morphologic residual disease at transplant | 8/27 (30) | 6/18 (33) | 2/9 (22) | 0.55 |
| Missing | 68 | 40 | 28 | |
| GvHD | 33/66 (50) | 18/38 (47) | 15/28 (54) | 0.62 |
| De novo AML | 78 (82) | 50 (86) | 28 (76) | 0.25 |
| Secondary AML | 17 (18) | 8 (14) | 9 (24) | 0.25 |
| Gene | Frameshifts | Non-frameshift variants |
|---|---|---|
| NPM1 | 26 | 0 |
| RUNX1 | 13 | 6 |
| TET2 | 7 | 7 |
| BCOR | 6 | 4 |
| ASXL1 | 4 | 3 |
| WT1 | 4 | 1 |
| STAG2 | 4 | 7 |
| ETV6 | 2 | 2 |
| ZRSR2 | 2 | 2 |
| CEBPA | 2 | 6 |
| TP53 | 2 | 9 |
| PHF6 | 1 | 6 |
| EZH2 | 1 | 3 |
| FLT3 | 28 | |
| NRAS | 19 | |
| DNMT3A | 19 | |
| IDH2 | 14 | |
| IDH1 | 12 | |
| PTPN11 | 11 | |
| SRSF2 | 10 | |
| CBL | 4 | |
| U2AF1 | 4 | |
| SETBP1 | 4 | |
| JAK2 | 3 | |
| SF3B1 | 3 | |
| JAK3 | 2 | |
| KIT | 2 |
| HR (log rank) FS vs. no-FS (CI) | P value (log rank) FS vs. no-FS | |
|---|---|---|
| FS: frameshift; HR: hazard ratio; CI: confidence interval. | ||
| Overall survival all patients | 0.56 (0.34 - 0.95) | 0.043 |
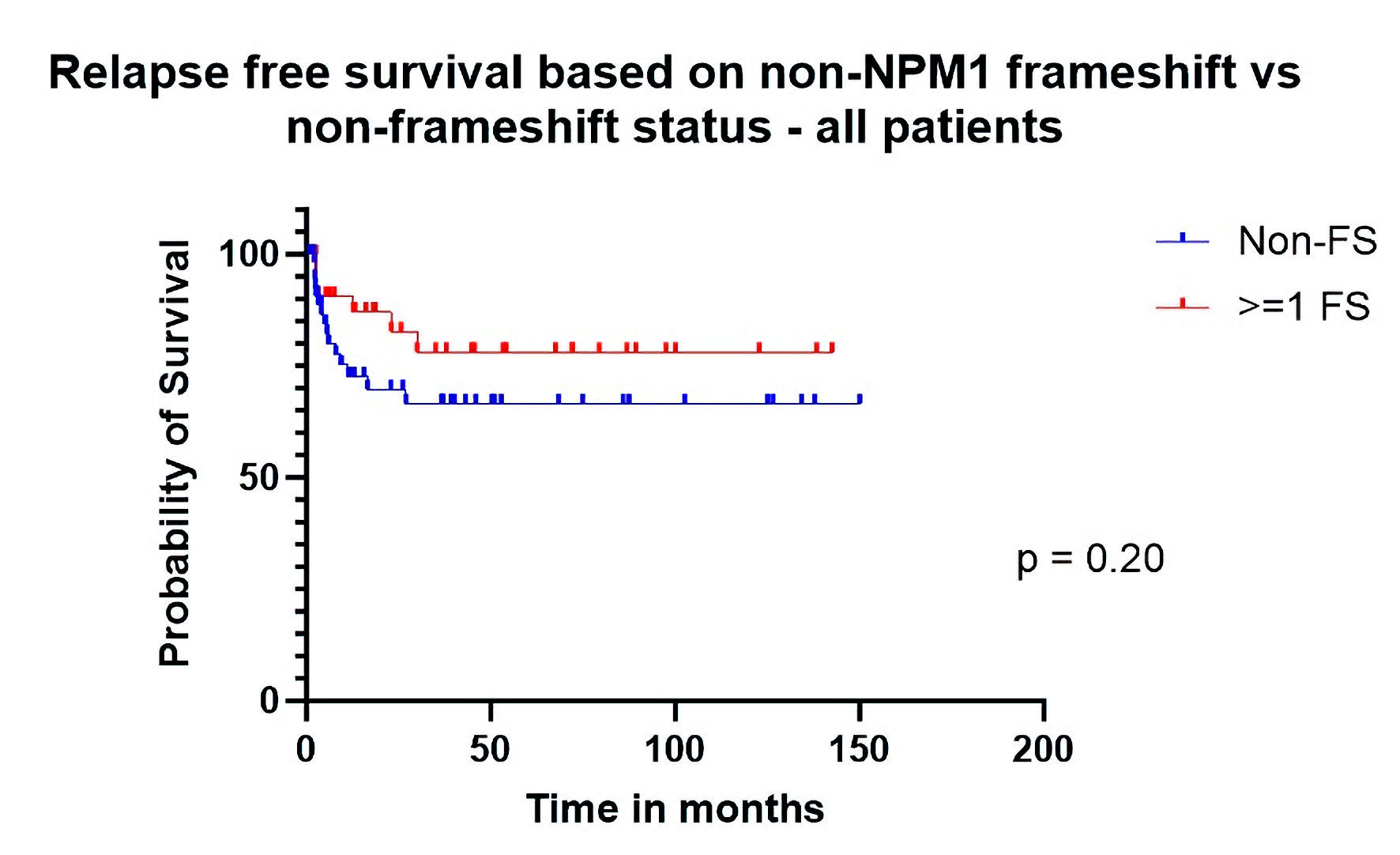
| Relapse-free survival all patients | 0.55 (0.23 - 1.30) | 0.2 |
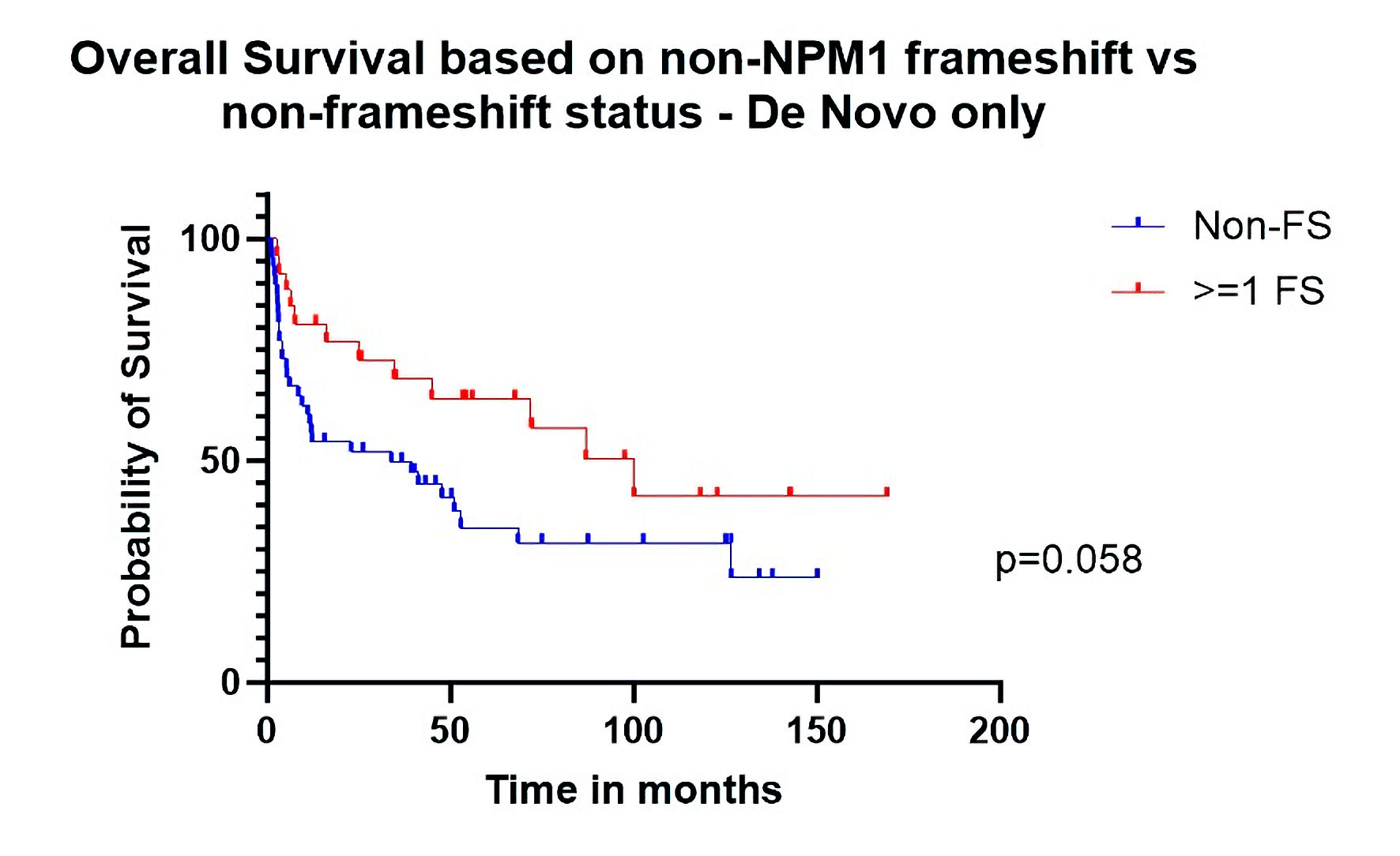
| Overall survival de novo only | 0.55 (0.30 - 1.02) | 0.058 |
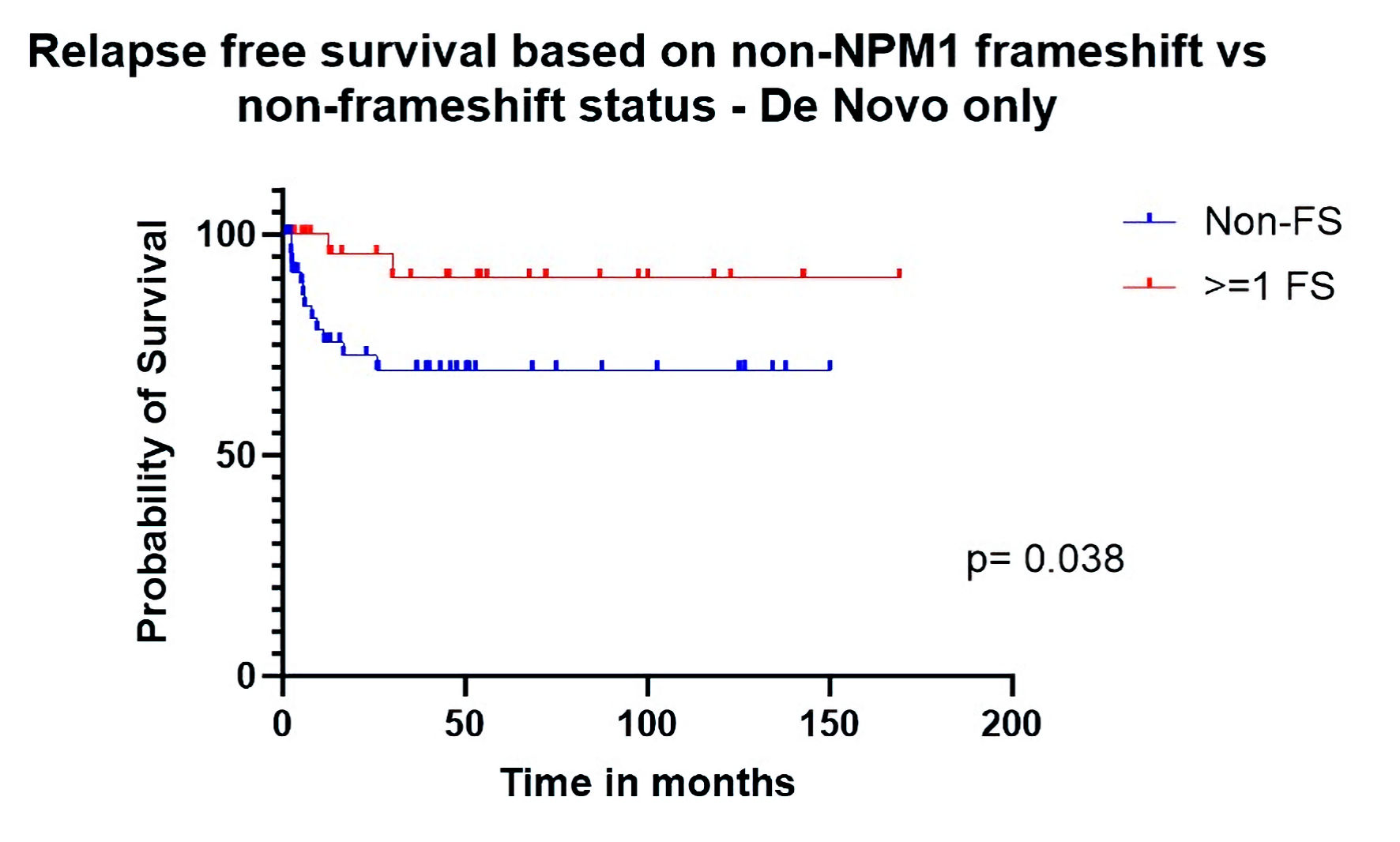
| Relapse-free survival de novo only | 0.24 (0.082 - 0.68) | 0.038 |
| Overall survival | Relapse | |||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| *P < 0.05. AML: acute myeloid leukemia; GvHD: graft-vs-host disease. | ||||
| All cases | ||||
| FS vs. no FS | 0.49 (0.26 - 0.87) | 0.018* | 0.42 (0.16 - 1.11) | 0.08 |
| Age | 1.01 (0.99 - 1.03) | 0.42 | 1 (0.97 - 1.03) | 0.94 |
| Disease risk group (NCCN) vs. intermediate | ||||
| Favorable | 0.98 (0.38 - 2.45) | 0.98 | 0 | 0.96 |
| Adverse | 1.19 (0.64 - 2.22) | 0.58 | 0.82 (0.29 - 2.3) | 0.9 |
| Secondary AML | 2.3 (1.18 - 4.46) | 0.014* | 4.12 (0.51 - 12.3) | 0.006* |
| GvHD positive | 1.43 (0.84 - 2.43) | 0.19 | 0.81 (0.32 - 2.07) | 0.66 |
| De novo only | ||||
| FS vs. no FS | 0.45 (0.22 - 0.92) | 0.028* | 0.16 (0.03 - 0.79) | 0.024* |
| Age | 1.01 (0.99 - 1.03) | 0.43 | 1 (0.96 - 1.03) | 0.985 |
| Disease risk group vs. intermediate | ||||
| Favorable | 1.01 (0.38 - 2.68) | 0.99 | 0 | 0.95 |
| Adverse | 1.09 (0.55 - 2.18) | 0.78 | 1.54 (0.51 - 4.7) | 0.45 |
| GvHD positive | 1.68 (0.91 - 3.1) | 0.1 | 1.28 (0.4 - 4.09) | 0.67 |