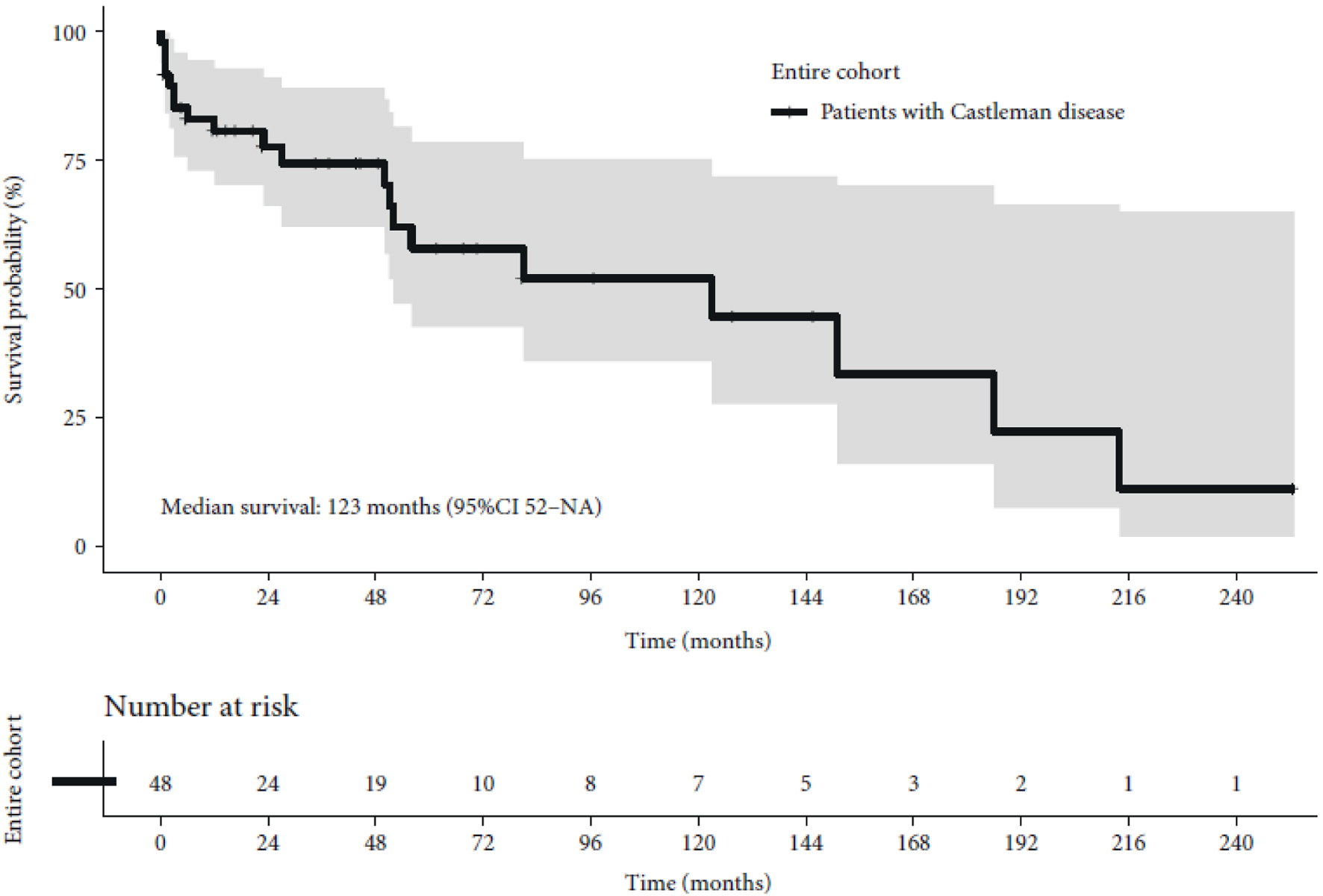
Figure 1. Kaplan-Meier curve of overall survival. CI: confidence interval; NA: not available.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Short Communication
Volume 13, Number 5, October 2024, pages 207-215
Siltuximab in Idiopathic Multicentric Castleman Disease: Real-World Experience
Figure

Tables
| Variable | Cohort | P-valuea | ||
|---|---|---|---|---|
| Overall (N = 48) | Greece (n = 28) | Romania (n = 20) | ||
| aPearson’s Chi-squared test; Wilcoxon rank sum exact test; Fisher’s exact test. CHOP: cyclophosphamide, doxorubicin, vincristine, prednisolone; CFA: complete Freund’s adjuvant; CVP: cyclophosphamide, vincristine, prednisolone; DEXA: dexamethasone; DOXO: doxorubicin; HHV-8: human herpesvirus-8; HIV: human immunodeficiency virus; R: rituximab; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; R-CVP: rituximab, cyclophosphamide, vincristine, prednisolone; SD: standard deviation. | ||||
| Sex, n/N (%) | 0.082 | |||
| Male | 33/48 (68.8) | 22/28 (78.6) | 11/20 (55.0) | |
| Female | 15/48 (31.3) | 6/28 (21.4) | 9/20 (45.0) | |
| Age (years), mean (SD) | 65 (19.0) | 74 (18.0) | 54 (14.0) | < 0.001 |
| Fever, n/N (%) | < 0.001 | |||
| Yes | 19/48 (39.6) | 17/28 (60.7) | 2/20 (10.0) | |
| No | 27/48 (56.3) | 9/28 (32.1) | 18/20 (90.0) | |
| Unknown | 2/48 (4.2) | 0/20 (0.0) | 2/28 (7.1) | |
| Night sweats, n/N (%) | 0.47 | |||
| Yes | 19/48 (39.6) | 12/28 (42.9) | 7/20 (35.0) | |
| No | 27/48 (56.3) | 14/28 (50.0) | 13/20 (65.0) | |
| Unknown | 2/48 (4.2) | 0/20 (0.0) | 2/28 (7.1) | |
| Weight loss, n/N (%) | 0.27 | |||
| Yes | 30/48 (62.5) | 19/28 (67.9) | 11/20 (55.0) | |
| No | 16/48 (33.3) | 7/28 (25.0) | 9/20 (45.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Asthenia, n/N (%) | 0.67 | |||
| Yes | 33/48 (68.8) | 19/28 (68.9) | 14/20 (70.0) | |
| No | 13/48 (27.1) | 7/28 (25.0) | 6/20 (30.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Threatened end organ function: bowel ischemia due to bowel obstruction from enlarged node, n/N (%) | 0.15 | |||
| Yes | 2/48 (4.2) | 0/28 (0.0) | 2/20 (10.0) | |
| No | 44/48 (91.7) | 26/28 (92.9) | 18/20 (90.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Threatened end organ function: renal failure/impairment due to ureteral obstruction by enlarged node, n/N (%) | 0.63 | |||
| Yes | 3/48 (6.2) | 2/28 (7.1) | 1/20 (5.0) | |
| No | 43/48 (89.6) | 24/28 (85.7) | 19/20 (95.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Threatened end organ function: liver impairment, n/N (%) | 0.50 | |||
| Yes | 7/48 (14.6) | 3/28 (10.7) | 4/20 (20.0) | |
| No | 39/48 (81.5) | 23/28 (82.1) | 16/20 (80.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Single mass, n/N (%) | 0.42 | |||
| Yes | 2/48 (4.2) | 0/20 (0.0) | 2/28 (7.1) | |
| No | 44/48 (91.7) | 20/20 (100.0) | 24/28 (85.7) | |
| Unknown | 2/48 (4.2) | 0/20 (0.0) | 2/28 (7.1) | |
| Two or more masses, n/N (%) | 0.17 | |||
| Yes | 40/48 (83.3) | 21/28 (75.0) | 19/20 (95.0) | |
| No | 6/48 (12.5) | 5/28 (17.9) | 1/20 (5.0) | |
| Unknown | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Splenomegaly, n/N (%) | 29/48 (60.4) | 9/20 (45.0) | 20/28 (71.4) | 0.065 |
| Method for splenomegaly assessment n/N, (%) | < 0.001 | |||
| Clinician | 6/48 (12.5) | 0/28 (0.0) | 6/20 (30.0) | |
| Radiology | 2/48 (4.2) | 0/28 (0.0) | 2/20 (10.0) | |
| Clinician and radiology | 37/48 (77.1) | 26/28 (92.9) | 11/20 (55.0) | |
| Unknown | 3/48 (6.2) | 2/28 (7.1) | 1/20 (5.0) | |
| Pleural or peritoneal serous effusion (irrespective of cell content), n/N (%) | 17/48 (35.4) | 12/28 (42.8) | 5/20 (25.0) | 0.20 |
| Anemia, n/N (%) | 27/48 (56.3) | 21/28 (75.0) | 6/20 (30.0) | 0.002 |
| Lymphopenia, n/N (%) | 12/48 (25.0) | 8/28 (28.6) | 4/20 (20.0) | 0.50 |
| Transformation to aggressive lymphoma, n/N (%) | 0.073 | |||
| Yes | 4/48 (8.3) | 4/28 (14.3) | 0/20 (0.0) | |
| No | 43/48 (89.6) | 24/28 (85.7) | 19/20 (95.0) | |
| Unknown | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| Malignancies, n/N (%) | 7/48 (14.6) | 5/28 (17.9) | 2/20 (10.0) | 0.68 |
| Autoimmune diseases, n/N (%) | 5/48 (10.4) | 3/28 (10.7) | 2/20 (10.0) | > 0.99 |
| Cardiovascular diseases, n/N (%) | 21/48 (43.8) | 14/28 (50.0) | 7/20 (35.0) | 0.30 |
| Diabetes, n/N (%) | 8/48 (16.7) | 7/28 (25.0) | 1/20 (5.0) | 0.12 |
| HHV-8 infections, n/N (%) | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | > 0.99 |
| HIV infections, n/N (%) | 2/48 (4.2) | 1/28 (3.6) | 1/20 (5.0) | > 0.99 |
| Hepatomegaly, n/N (%) | 0.27 | |||
| Yes | 24/48 (50.0) | 14/28 (50.0) | 10/20 (50.0) | |
| No | 22/48 (45.8) | 14/28 (50.0) | 8/20 (40.0) | |
| Unknown | 2/48 (4.2) | 0/28 (0.0) | 2/20 (10.0) | |
| Gastric manifestation, n/N (%) | 4/48 (8.3) | 4/28 (14.3) | 0/20 (0.0) | 0.13 |
| Thyroid manifestation, n/N (%) | 3/48 (6.2) | 3/28 (10.7) | 0/20 (0.0) | 0.26 |
| Pancreas manifestation, n/N (%) | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | 0.42 |
| Hepatic manifestation, n/N (%) | 5/48 (10.4) | 2/28 (7.1) | 3/20 (15.0) | 0.64 |
| Renal manifestation, n/N (%) | 4/48 (8.3) | 2/28 (7.1) | 2/20 (10.0) | > 0.99 |
| Pulmonary manifestation, n/N (%) | 16/48 (33) | 12/28 (42.9) | 4/20 (20.0) | 0.10 |
| Radiation therapy, n/N (%) | 0.046 | |||
| Yes | 3/48 (6.2) | 1/28 (3.6) | 2/20 (10.0) | |
| No | 37/48 (77.1) | 25/28 (89.3) | 12/20 (60.0) | |
| Unknown | 8/48 (16.7) | 2/28 (7.1) | 6/20 (30.0) | |
| Previous therapeutic strategies, n/N (%) | 0.030 | |||
| CFA-DEXA | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| CHOP | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| Corticosteroids | 4/48 (8.3) | 3/28 (10.7) | 1/20 (5.0) | |
| CVP | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| CVP, R-CHOP | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| CVP/Etoposide | 3/48 (6.2) | 0/28 (0.0) | 3/20 (15.0) | |
| Melphalan-Vc-methylprednisolone | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| None | 3/48 (6.2) | 3/28 (10.7) | 0/20 (0.0) | |
| R-CFA-DOXO-PREDNISON | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| R-CHOP | 11/48 (22.9) | 8/28 (28.6) | 3/20 (15.0) | |
| R-CHOP/etoposide | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| R-CVP | 5/48 (10.4) | 4/28 (14.3) | 1/20 (5.0) | |
| R-CVP + R maintenance | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| R-CVP/etoposide | 2/48 (4.2) | 0/28 (0.0) | 2/20 (10.0) | |
| Rituximab | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| Siltuximab | 10/48 (20.8) | 3/28 (10.7) | 7/20 (35.0) | |
| Tocilizumab | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| Number of prior lines of treatment before siltuximab, n/N (%) | 0.006 | |||
| 0 | 3/48 (6.2) | 3/28 (10.7) | 0/20 (0.0) | |
| 1 | 36/48 (75.0) | 16/28 (57.1) | 20/20 (100.0) | |
| 2 | 6/48 (12.5) | 6/28 (21.4) | 0/20 (0.0) | |
| 3 | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| Unknown | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| Number of siltuximab cycles, n/N (%) | 0.10 | |||
| 0 | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| 1 | 7/48 (14.6) | 5/28 (17.9) | 2/20 (10.0) | |
| 2 | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| 4 | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| 6 | 11/48 (22.9) | 4/28 (14.3) | 0/20 (0.0) | |
| 7 | 4/48 (8.3) | 1/28 (3.6) | 3/20 (15.0) | |
| 8 | 8/48 (16.7) | 6/28 (21.4) | 2/20 (10.0) | |
| 9 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 10 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 12 | 2/48 (4.2) | 2/28 (7.1) | 0/20 (0.0) | |
| 14 | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| 16 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 17 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 18 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 24 | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| 33 | 1/48 (2.1) | 0/28 (0.0) | 1/20 (5.0) | |
| 48 | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | |
| Unknown | 3/48 (6.2) | 3/28 (10.7) | 0/20 (0.0) | |
| Variable | N | Cohort | P-valuea | ||
|---|---|---|---|---|---|
| Overall (N = 48) | Greece (n = 28) | Romania (n = 20) | |||
| aFisher’s exact test. bResponse data were unknown for 10 patients. AE: adverse event. | |||||
| Best response, n/N (%) | 38b | 0.17 | |||
| Complete response | 21/38 (55.3) | 10/19 (52.6) | 11/19 (57.9) | ||
| Partial response | 6/38 (15.8) | 3/19 (15.8) | 3/19 (15.8) | ||
| No response or stable disease | 6/38 (15.8) | 4/19 (21.1) | 2/19 (10.5) | ||
| Progressive disease | 5/38 (13.2) | 2/19 (10.5) | 3/19 (15.8) | ||
| Disease status at last known follow-up, n/N (%) | 48 | 0.031 | |||
| Disease progression | 19/48 (39.6) | 14/28 (50.0) | 5/20 (25.0) | ||
| Progression free | 23/48 (47.9) | 9/28 (32.1) | 14/20 (70.0) | ||
| Unknown | 6/48 (12.5) | 5/28 (17.9) | 1/20 (5.0) | ||
| Survival status at last known follow-up, n/N (%) | 48 | 0.003 | |||
| Alive | 28/48 (58.3) | 11/28 (39.3) | 17/20 (85.0) | ||
| Dead | 19/48 (39.6) | 16/28 (57.1) | 3/20 (15.0) | ||
| Unknown | 1/48 (2.1) | 1/28 (3.6) | 0/20 (0.0) | ||
| Primary cause of death, n/N (%) | 48 | 0.029 | |||
| Death from AE | 3/48 (6.2) | 3/28 (10.7) | 0/20 (0.0) | ||
| Death from malignant disease under study | 6/48 (12.5) | 5/28 (17.9) | 1/20 (5.0) | ||
| Death from other causes | 10/48 (20.8) | 8/28 (28.6) | 2/20 (10.0) | ||
| Alive | 29/48 (60.4) | 12/28 (42.9) | 17/20 (85.0) | ||
| Adverse event | n (%) |
|---|---|
| ALT: alanine aminotransferase; AST: aspartate aminotransferase. | |
| Raised ALT, AST or bilirubin level | 5 (10.4) |
| Anxiety | 5 (10.4) |
| Allergic reactions | 3 (6.3) |
| Nausea/diarrhea | 3 (6.3) |
| Anemia | 2 (4.2) |
| Thrombocytopenia | 2 (4.2) |
| Hypertension | 2 (4.2) |
| Bleeding | 1 (2.1) |
| Atrial fibrillation | 1 (2.1) |