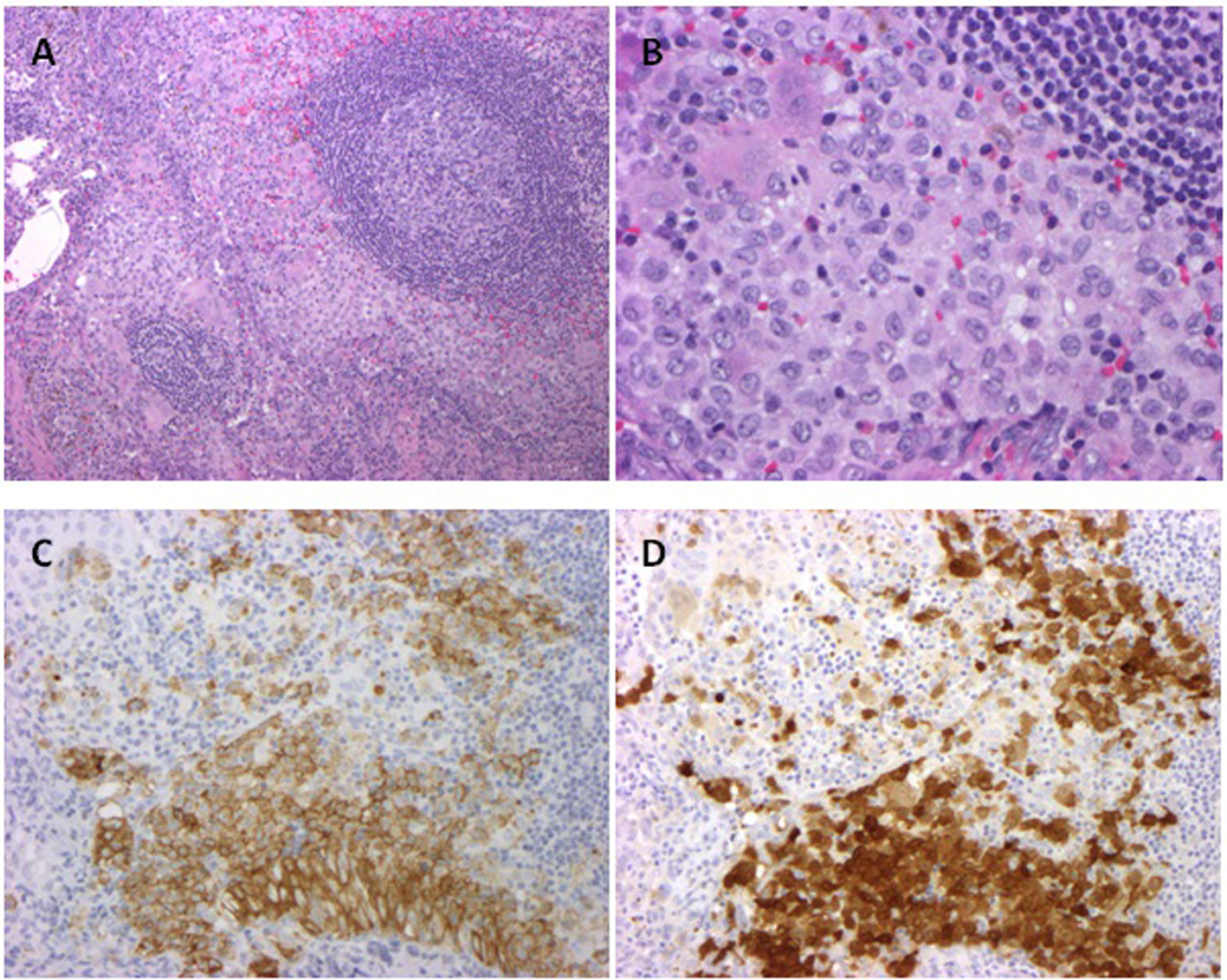
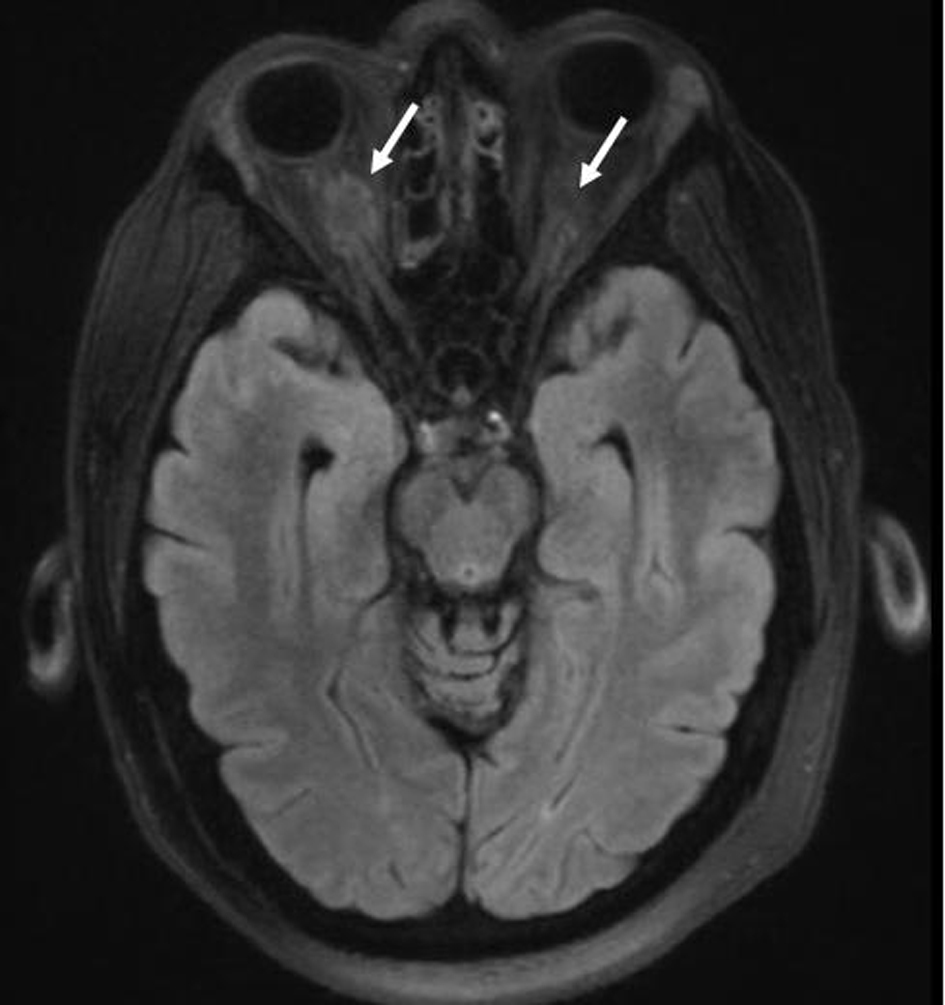
| Summary of pediatric studies for management of MS-LCH |
| LCH-I: Gadner et al 2001 [4] | 143 | 24 weeks:
Vinblastine (V) or etoposide (E)
plus single initial dose of corticosteroids | At 6 weeks
V: 57%
E: 49% | V: 47%
E: 58%
| 3 years
V: 61%
E: 55% | 3 years
V: 76%
E: 83% |
| LCH-II: Gadner et al 2008 [5] | 193 | Arm A: prednisone and vinblastine × 6 weeks + vinblastine/prednisone + 6MCP × 18 weeks
Arm B: arm A + etoposide (for 24 weeks) | At 6 weeks
Arm A: 63%
Arm B: 71% | NA | 3 years
Arm A: 46%
Arm B: 46% | 5 years
Arm A: 74%
Arm B: 79% |
| LCH-III: Gadner et al 2013 [6] | 235
RO+ | 6 weeks × 1 - 2 cycles:
Arm A: vinblastine + prednisone
Arm B: vinblastine + prednisone + MTX
12 months:
Arm A: vinblastine + prednisone + 6MCP
Arm B: vinblastine + prednisone + MTX + 6MCP | At 6 weeks
Arm A: 65%
Arm B: 66% | Arm A: 30%
Arm B: 46% | 3 years
Arm A: 25%
Arm B: 29% | 5 years
Arm A: 87%
Arm B: 82% |
| Japan LCH Study Group (JLSG-96): Morimoto et al 2006 [7] | 59 | 6 weeks:
Ara-C + VCR + prednisolone
Response: 6 months maintenance tx
Poor response: salvage therapy | 76.30% | NA | 45.30% | 5 years
94.40% |
| Summary of adult studies for management of MS-LCH |
| Morimoto et al 2013 [8] | 14 (10 MS) | Vinblastine/prednisolone + MTX + 6MCP × 36 weeks | 60% | | NA | 80% |
| Von Stebut et al 2008 [9] | 1 | Prednisolone + vinblastine + 6MCP × 12 months | 100% | | NA | NA |
| Matsuki et al 2011 [10] | 2 | Prednisone + vinblastine + 6-MCP × 6 - 9 months | 100% | | NA | NA |
| Adam et al 2012 [11] | 10 (8 MS) | Cladribine × 4 - 6 cycles
No response → cladribine + cyclophosphamide + dexamethasone | 90% | | NA | NA |
| Pardanani et al 2003 [12] | 5 | Cladribine × 4 cycles | 100% | | NA | NA |
| Derenzini et al 2010 [13] | 7 (3MS, 4 SS) | MACOP-B: methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone and bleomycin | 100% | | 67% | 33% |