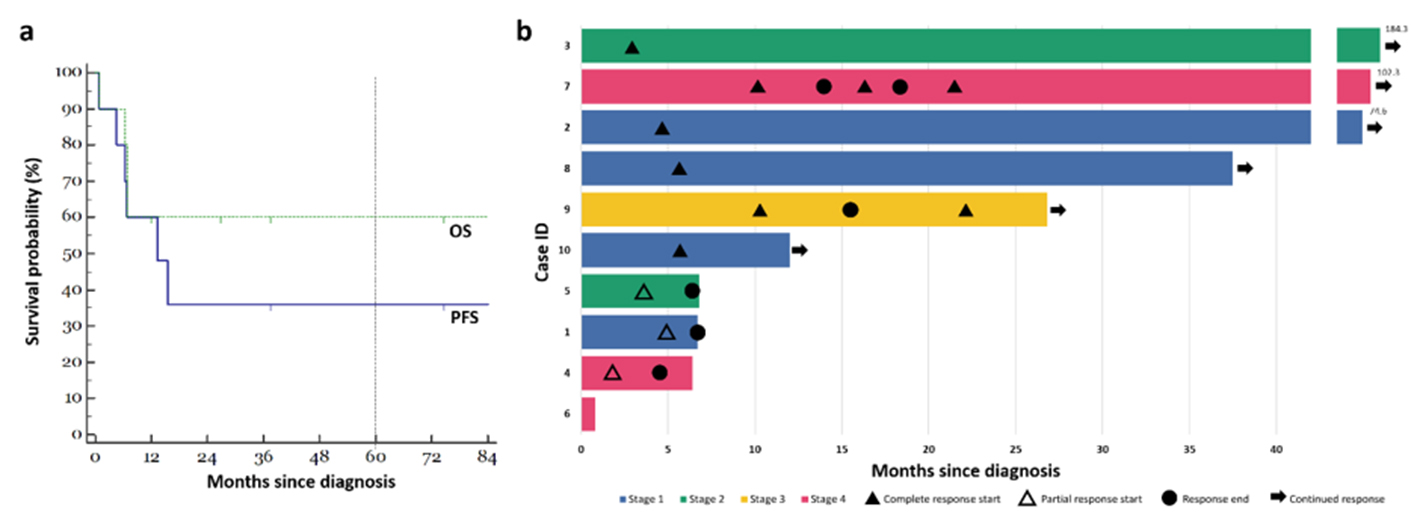
Figure 1. Clinical course of patients with plasmablastic lymphoma. (a) Kaplan-Meier estimates for our cohort of 10 patients. The green line indicates OS, while the blue line indicates PFS. Five-year absolute survival estimates were given at 60% and 36% for OS and PFS respectively. (b) Swimmer plot illustrating a summary of the clinical courses taken by each of the 10 patients in our cohort. OS: overall survival; PFS: progression-free survival.
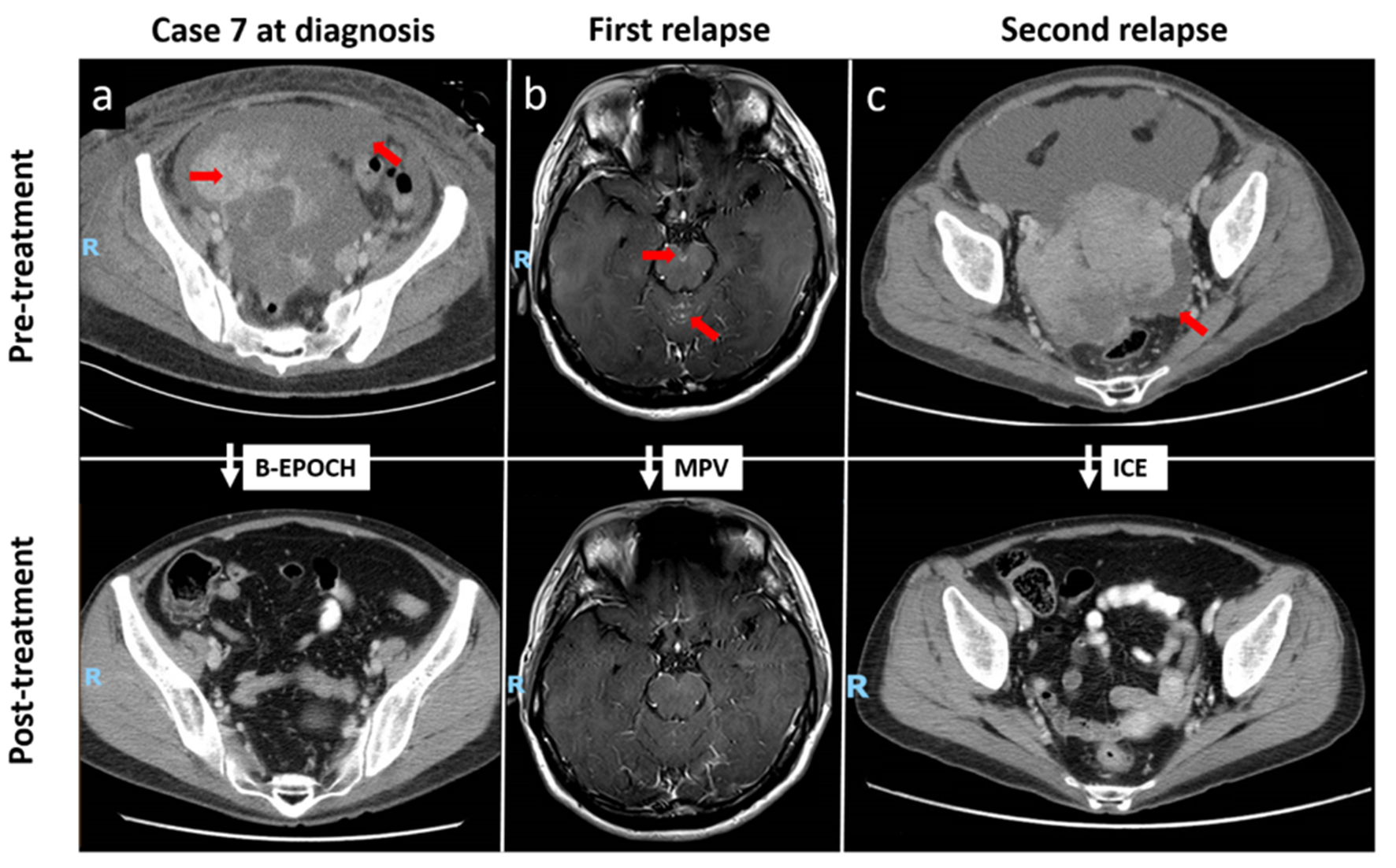
Figure 2. Repeated CRs to chemotherapy in a patient with relapsed stage IV plasmablastic lymphoma (case 7). (a) Contrast-enhanced CT illustrating CR of multiple solid cystic masses and peritoneal nodules following B-EPOCH as first-line therapy. (b) Contrast-enhanced T1-weighted MRI showing abnormal leptomeningeal enhancement at the cerebellar vermis and on the surface of the midbrain at level of the interpeduncular cistern, correlating with the presence of malignant blast cells on cerebrospinal fluid examination. Resolution of disease was observed following MPV as second-line therapy. (c) CR of enhancing soft-tissue masses in the pelvic cavity following third-line chemotherapy with the ICE regimen. Red arrows indicate initial sites of disease. CRs: complete responses; CT: computed tomography; B-EPOCH: bortezomib plus etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin; MRI: magnetic resonance imaging; MPV: methotrexate, procarbazine, vincristine; ICE: ifosfamide, carboplatin, etoposide.
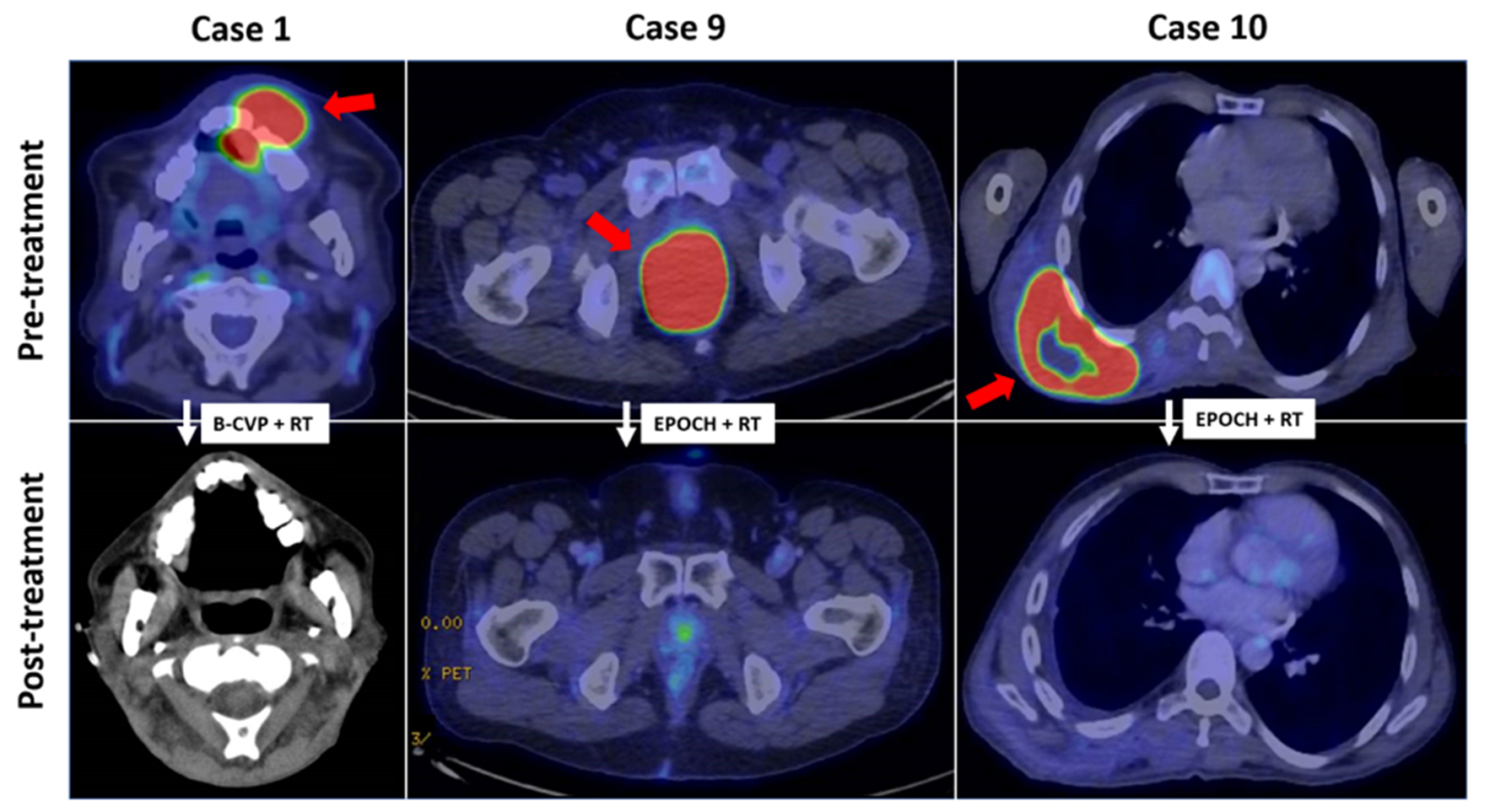
Figure 3. FDG-PET/CT imaging features in plasmablastic lymphoma. (case 1) FDG-avid soft tissue mass (SUVmax 21.5) in the oral cavity in partial response following B-CVP and consolidation radiotherapy. (case 9) Locally-advanced FDG-avid anorectal mass (SUVmax 30.9) in complete metabolic remission after EPOCH and consolidation radiotherapy. (case 10) Complete metabolic response of initially FDG-acid posterior chest wall mass (SUVmax 28.2) after EPOCH and radiotherapy. Red arrows indicate initial sites of disease. FDG-PET/CT: 18-fluorodeoxyglucose positron emission tomography/computed tomography; B-CVP: bortezomib, cyclophosphamide, vincristine, prednisone; EPOCH: etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin.


