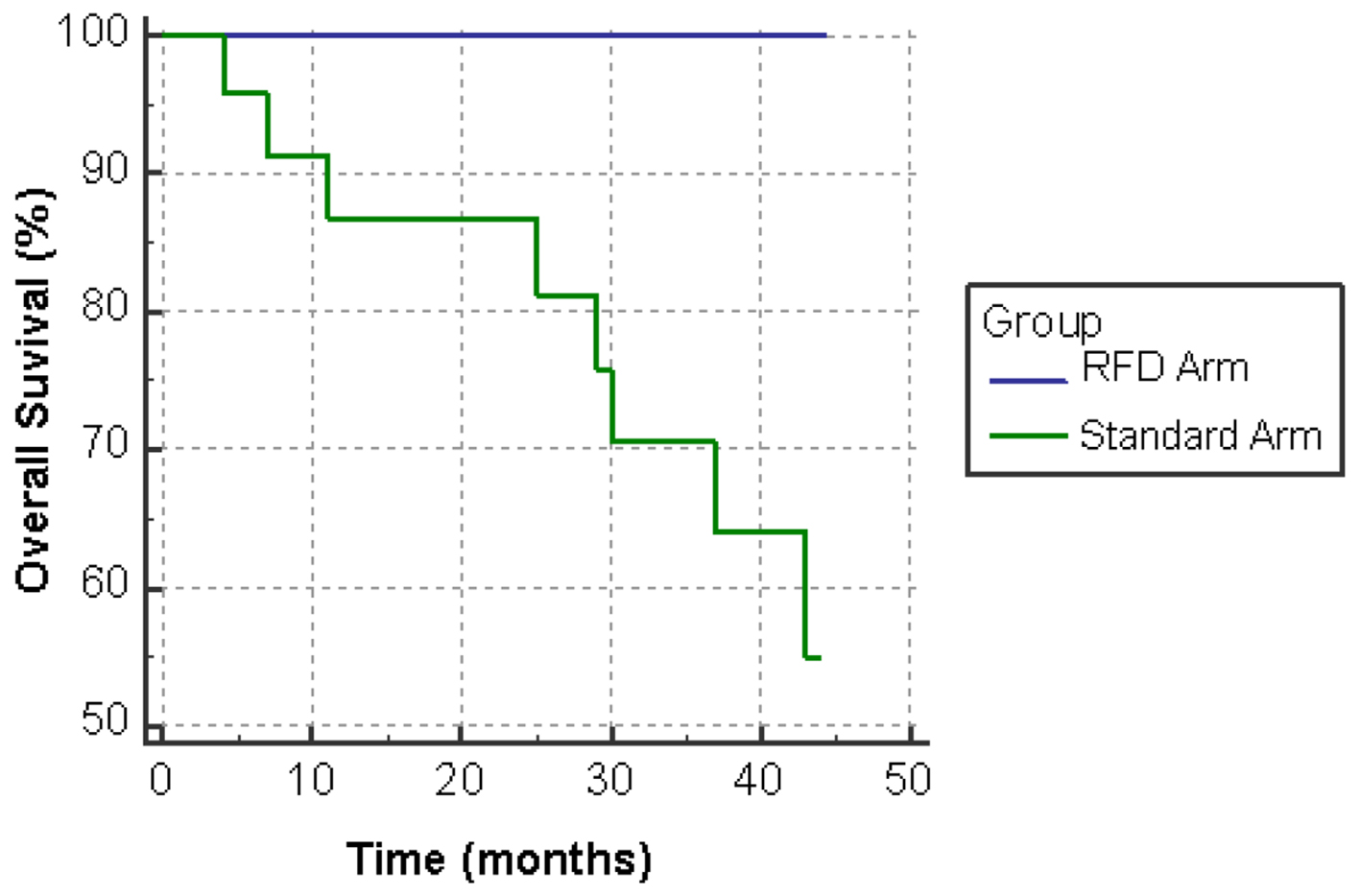
Figure 1. Overall survival (OS) on standard dose versus reduced frequency dosing (RFD) ibrutinib.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 9, Number 3, September 2020, pages 55-61
Outcomes of Reduced Frequency Dosing of Ibrutinib in Chronic Lymphocytic Leukemia Patients Following Complete or Partial Remission: A Pilot Study
Figures

Tables
| CLL: chronic lymphocytic leukemia; RFD: reduced frequency dosing. | ||
| Forty CLL patients on ibrutinib | Twenty-two (55%) continued therapy | Sixteen (40%) on RFD thrice weekly regimen (three with p53 mutation) |
| Six (15%) continued on standard dose (two with p53 mutation) | ||
| Eighteen (45%) ceased therapy | Seven (17.5%) died (five from Richter’s transformation with p53) | |
| Four (10%) required other therapies | ||
| Four (10%) have not required other therapy | ||
| Three (7.5%) lost to follow-up or refused therapy | ||
| Side effects | All patients in audit (n = 40) | Reduced frequency dosing arm (n = 16) | RESONATE (n = 195) | |
|---|---|---|---|---|
| Prior to switchover | After switchover | |||
| Median follow-up (months) | 34 | 20.5 | 21 | 19 |
| Diarrhea | 4 (10%) | 1 (6.25%) | 0 | 105 (53.8%) |
| Bleeding | 5 (12.5%) | 1 (6.25%) | 0 | 84 (43%) 19 (10%) > 18 m |
| Fatigue | 14 (35%) | 6 (37.5%) | 4 (25%) | 67 (34.4%) |
| Nausea | 2 (5%) | 1 (6.25%) | 0 | 61 (31.3%) |
| Pyrexia | 4 (10%) | 0 | 0 | 58 (29.7%) |
| Cough | 20% (8) | 0 | 0 | 51 (26.2%) |
| Neutropenia | 2 (5%) | 0 | 0 | 50 (25.6%) |
| Anemia | 2 (5%) | 0 | 0 | 49 (25.1%) |
| Upper respiratory tract infection | 10 (25%) | 2 (12.5%) | 1 (6.25%) | 49 (25.1%) |
| Peripheral edema | 0 | 0 | 0 | 38 (19.5%) |
| Sinusitis | 0 | 0 | 0 | 37 (19.0%) |
| Arthralgia | 4 (10%) | 0 | 0 | 36 (18.5%) |
| Muscle spasms | 0 | 0 | 0 | 36 (18.5%) |
| Constipation | 2 (5%) | 0 | 0 | 35 (17.9%) |
| Headache | 4 (10%) | 2 (12.5%) | 2 (12.5%) | 33 (16.9%) |
| Pneumonia | 2 (5%) | 0 | 0 | 33 (16.9%) |
| Thrombocytopenia | 1 (2.5%) | 0 | 0 | 33 (16.9%) |
| Vomiting | 0 | 0 | 0 | 33 (16.9%) |
| Hypertension | 1 (2.5%) | 1 (6.25%) | 0 | 10 (5%) 4 (2%) > 18 m |
| Atrial fibrillation | 4 (10%) | 2 (12.5%) | 1 (6.25%) | 8 (4%) 0 > 18 m |
| Skin/nail lesions | 14 (35%) | 3 (18.75%) | 1 (6.25%) | Not reported |