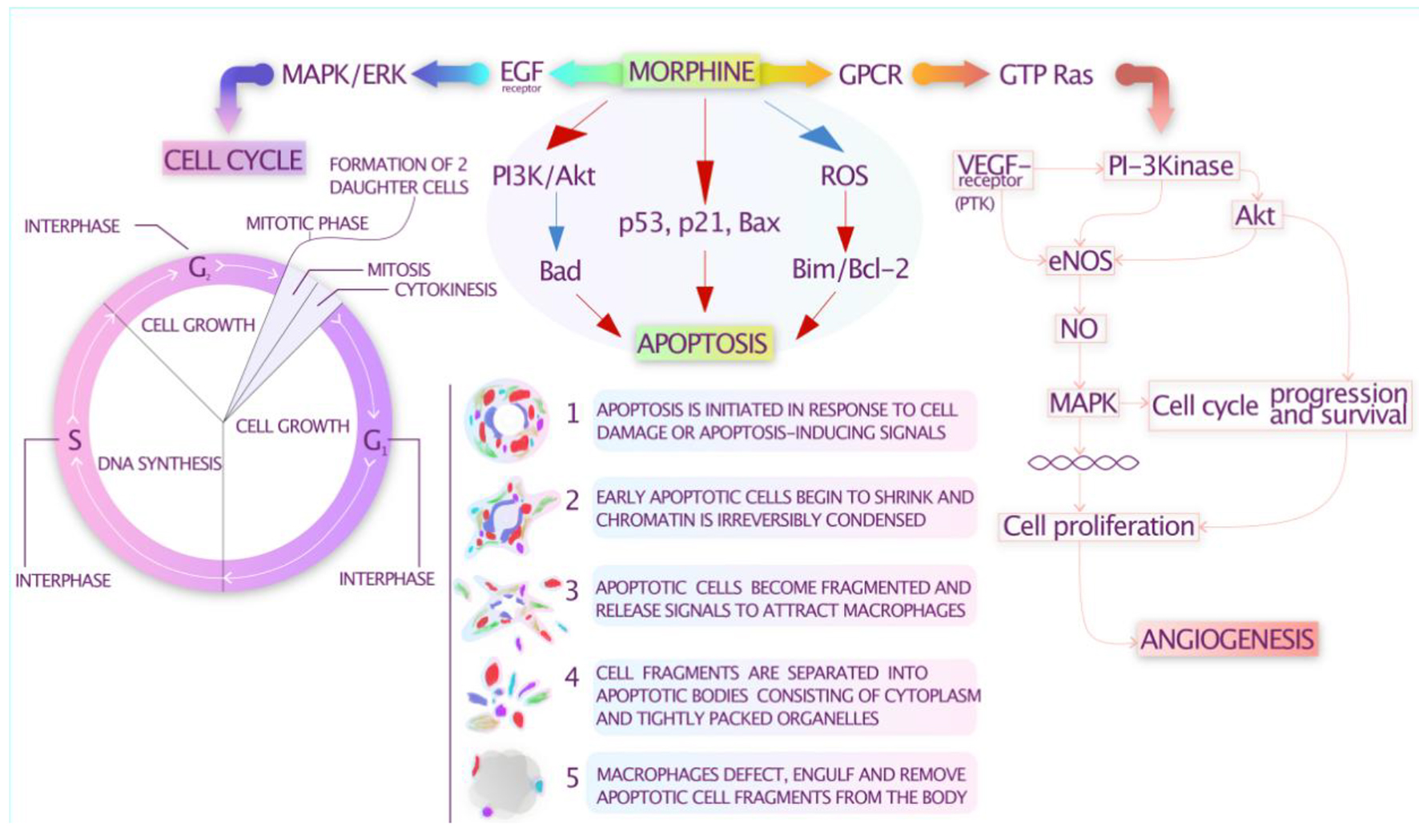
Figure 1. Potential immunomodulatory effects of opioids.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 9, Number 3, September 2020, pages 41-54
Impact of Opioid Use in Hematological Malignancies: Clinical, Immunological and Concomitant Aspects
Figures

Table
| Authors, year | Study goal | Study description | Findings |
|---|---|---|---|
| Gaspani et al, 2002 [35] | Analysis of tramadol’s ability to stimulate NK cell activity and prevent metastasis progression in rodents | Experimental surgery was conducted with subsequent analysis of: 1) tramadol’s ability to prevent the effects of laparotomy on NK activity, and 2) the progression of the metastatic process of the NK-sensitive tumor model MADB106 in rodents. | Compared to morphine, the administration of tramadol (20 and 40 mg/kg) before and after surgery was associated with decreased progression of lung metastasis and preserved NK activity in post-operative animals. |
| Shavit et al, 2004 [36] | Analysis of the effects of different doses of fentanyl, administered at different time points relative to tumor inoculation, on natural killer cell cytotoxicity (NKCC) and on tumor metastasis in rats | Three hundred forty-four (344) rats were injected with low or high doses of fentanyl, 6 or 2 h before, simultaneously with or 1 h after being inoculated intravenously with MADB106 experimental tumor cells. Following the surgery, lung tumor retention, the severity of the metastatic process and NK cell activity were assessed at different time points. | Fentanyl administration was associated with impaired NKCC and increased risk of tumor metastasis. |
| Provinicali et al, 1991 [37] | The effect of morphine on the activity of NK and lymphokine-activated killer (LAK) cells in cancer patients | Twenty (20) patients with various forms of cancer were studied. At the end of a month of morphine administration, test results were compared with the results of healthy volunteers. | A significant decrease in NK activity and an increase in LAK activity were observed. |
| Provinicali et al, 1996 [38] | To study the effects of short-term and long-term administration of morphine on the activity of NK and LAK cells | Eighteen (18) patients with cancer of various natures were studied. Ten (10) patients received morphine, but eight patients did not. Changes were evaluated 30 min after a single injection of 10 mg of morphine and 1 month after daily administration of 90 mg of morphine. | Short-term effect: decrease in NK activity, increase in LAK activity, no change in the number of lymphocytes in peripheral blood. Long-term effect: an even greater decrease in NK activity and an increase in LAK activity, an increase in the content of CD3+ and CD4+ lymphocytes, a decrease in CD16+ lymphocytes. |
| Sacerdote et al,1997 [39] | Analysis of the potential immunosuppressive effects of morphine and morphine-derived drugs (codeine, hydromorphone, oxycodone) | Effects of listed agents on immune parameters (splenocyte proliferation, NK cell activity and interleukin-2 (IL-2) production) were evaluated in the mouse model. | Morphine had a potent immunosuppressive effect; codeine possessed limited immunosuppressive activity; hydromorphone and oxycodone were devoid of immunosuppressive effects. The pure antagonists naloxone and naltrexone potentiated immune responses. |
| Makimura et al, 2011 [40] | Analysis of cytokine levels as a possible predictor of morphine resistance | Forty-four (44) cancer patients received morphine according to the standard protocol for 8 days. | The study revealed no correlation between the administration of morphine and immune system functions. |
| Hashiguchi et al, 2005 [41] | Analysis of possible morphine effects on immune system function in cancer patients | Fourteen (14) patients were split into two groups. Group 1 contained patients who had not received morphine previously. The final dose of morphine was 20 - 30 mg by oral or intravenous administration. Group 2 contained patients who received morphine previously for a month. The initial dose of morphine was 40 - 120 mg. The final dose of morphine was 20 - 240 mg, by oral, intravenous, subcutaneous, rectal administration. | In group 1, there was a negative correlation with the level of immunoglobulins and proliferation of lymphocytes induced by phytohemagglutinin, but not with NK activity or the ratio of CD4/CD8 cells. In group 2, no correlations were found. |
| Palm et al, 1998 [42] | Analysis of the possible effects of morphine on immune function when administered orally | Ten (10) patients received morphine at a dose of 30 - 240 mg per day. Tests were taken before the start of morphine administration, as well as 1, 4 and 12 weeks after the start of morphine administration. | There was no change in the total number of lymphocytes or their populations, the rate of lymphocyte proliferation induced by phytohemagglutinin or the levels of IgM and IgG. Elevated levels of IL-2 were secreted by lymphocytes. |
| Sacerdote et al, 2000 [43] | Authors explored the impact of morphine and tramadol on pain and the immune system in 30 patients undergoing surgery for uterine carcinoma. | T lymphocyte proliferation and NK activity were assessed at different time points: before and after surgery, and 2 h after the IM administration of 10 mg of morphine (group 1) or 100 mg tramadol (group 2) for pain. | Tramadol and morphine showed comparable analgesic activity. However, in group 1, proliferative values remained lower than basal levels for 2 h after treatment, whereas in tramadol-treated patients proliferative values returned to basal levels. Tramadol, in contrast to morphine, enhanced the activity of NK cells. |
| Franchi et al, 2007 [44] | Analyzed the ability of buprenorphine to prevent the effects of surgery on HPA activation, NK activity and lung diffusion of the NK-sensitive tumor MADB106 | Buprenorphine (0.1 mg/kg) was compared with equianalgesic doses of 1) fentanyl (0.1 mg/kg) and 2) morphine (10 mg/kg) in an animal model. | In normal animals, morphine and fentanyl stimulate the HPA axis, decrease NK activity and trigger tumor metastasis, while buprenorphine is devoid of these effects. Buprenorphine, in contrast to other agents, was able to prevent the HPA and immune system alterations and ameliorate the increase of tumor metastasis induced by surgery-induced stress reaction. |
| Desmond et al, 2015 [45] | To verify whether anesthetic technique influences the distribution of NK cells, T lymphocytes and macrophages in intra-tumoral tissue and predict prognosis and response to therapy | Breast cancer patients were randomized to receive either a propofol-paravertebral anesthetic with continuing analgesia (PPA) or a standardized general anesthesia with opioid analgesia (GA) for 24 h post-operatively. | PPA induces increased levels of NK and T helper cell infiltration into breast cancer tissue compared with GA but not T suppressor cells or macrophages. Lack of immune cell infiltration could negatively affect the protective anti-tumor immunity. |
| Shen et al, 2014 [46] | To analyze the effects of morphine +/- flurbiprofen as post-operative analgesics on the immune systems of patients undergoing gastric cancer surgery | Sixty (60) patients undergoing gastric cancer surgery were randomized into two groups based on post-operative intravenous (IV) analgesia using morphine either with or without flurbiprofen. | Analgesia combined with morphine and flurbiprofen ameliorates the immune depression in T-lymphocyte subtypes and NK cells, while providing a similar analgesic effect to morphine alone. |
| Gong et al, 2014 [47] | Evaluation of effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T-cell frequencies | Authors compared the immunosuppressive effects of sufentanil and fentanyl on CD4+CD25+Foxp3+ regulatory T-cell (Tregs) frequencies both in vitro and in breast cancer (BC) patients undergoing eradicative operation. | Treg cells can inhibit anti-tumor immune responses. Sufentanil is more powerful than fentanyl in increasing the quantity of Tregs in vitro. Both agents have very similar analgesic potential; no significant differences in Treg frequencies between sufentanil and fentanyl noted. |