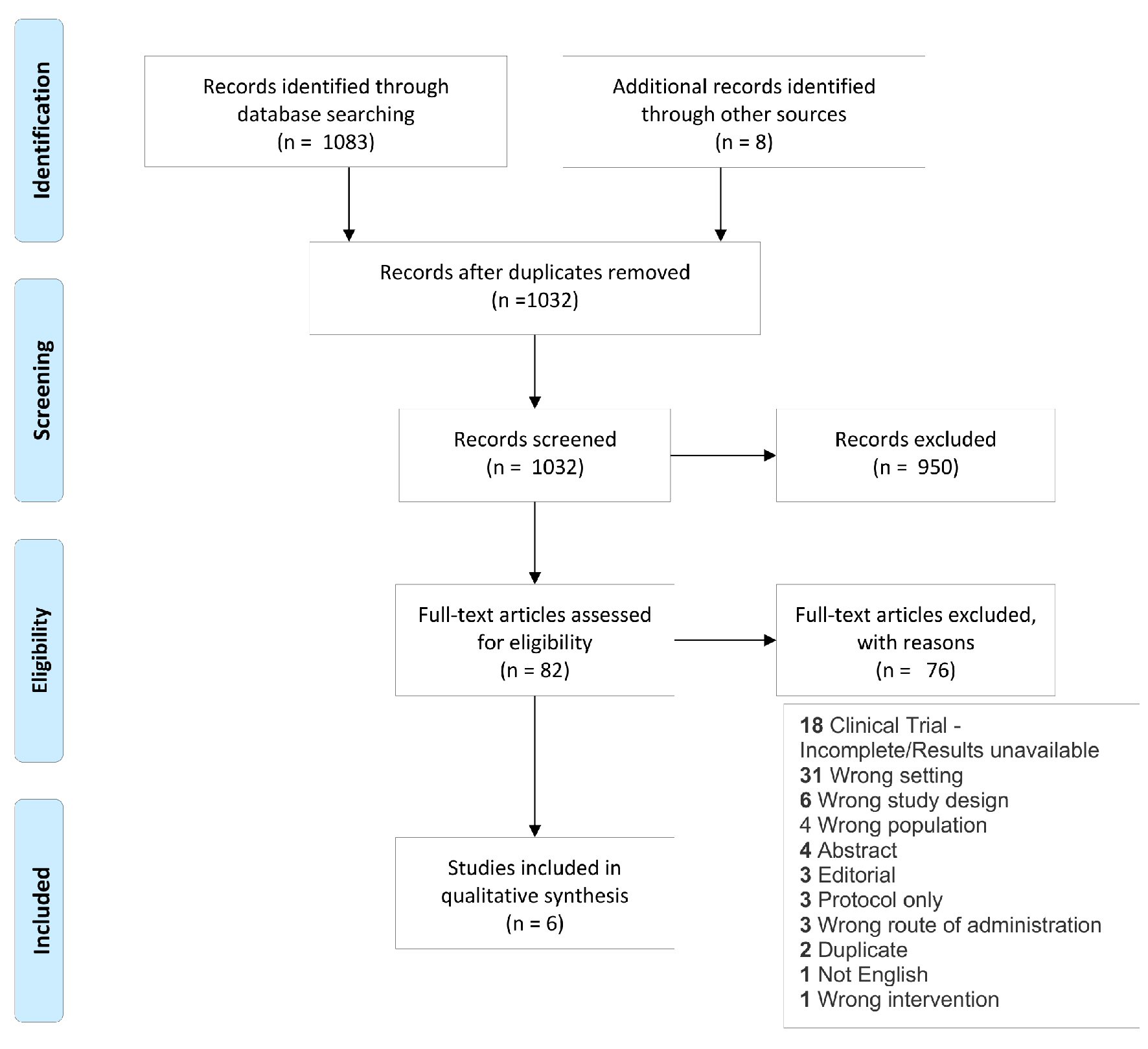
Figure 1. Study flow diagram showing literature selection criteria used for the systematic review.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 9, Number 4, December 2020, pages 97-108
The Impact of Preoperative Intravenous Iron Therapy on Perioperative Outcomes in Cardiac Surgery: A Systematic Review
Figure

Tables
| Author (location) | Cladellas et al, 2012 [32] (Spain) | Urena et al, 2017 [34] (Canada) | Johansson et al, 2015 [36] (Denmark) | Padmanabhan et al, 2018 [35] (UK) | Garrido-Martin et al, 2012 [33] (Spain) | Spahn et al, 2019 [37] (Switzerland) | |
|---|---|---|---|---|---|---|---|
| aWHO criteria (Hb < 13.0 g/dL in men or < 12.0 g/dL in women). bIV rhEPO at 500 IU/kg/day every week for 4 weeks and the fifth dose 48 h before VR with each session receiving IV iron sucrose (weight-based dosing). cTwo weight-based doses of subcutaneous EPO (0.75 µg/kg darbepoetin alfa). AF: atrial fibrillation; AKI: acute kidney injury; ARF: acute renal failure; CABG: coronary artery bypass grafting; CKMB: creatinine kinase-MB; CPB: cardiopulmonary bypass; CVA: cerebrovascular accident; ECG: electrocardiogram; EPO: erythropoietin; Hb: hemoglobin; HD: hemodialysis; HF: heart failure; IRF: immature reticulocyte fraction; LOS: length of stay; MCV: mean corpuscular volume; MI: myocardial infarction; PO: per os; QOL: quality of life; TAVI: trans-aortic valve implantation; TSAT: transferrin saturation; VR: valve replacement; pre-op: preoperatively; re-op: re-operation; BID: twice a day. | |||||||
| Design | Single center non-blinded non-randomized prospective cohort with historical control | Single center randomized double-blind prospective cohort | Single center randomized double-blind prospective cohort | Single center non-blinded randomized prospective cohort | Single center randomized double-blind prospective cohort | Single center randomized, double-blind prospective cohort | |
| Population | Mean age (years) | 72 | 81 | 65 | 74 | 65 | 68 |
| Anemia | Yesa | Yesa | Noa | Yesa | No | Yesa | |
| Surgery type | VR | VR, TAVI | CABG, VR | CABG, VR | CABG, VR | CABG, VR | |
| N | 134 | 100 | 60 | 50 | 210 | 484 | |
| Intervention | IV iron | Iron sucrose | Iron sucrose | Iron isomaltose | Ferric carboxymaltose | Iron sucrose | Ferric carboxymaltose |
| Dose | 200 mg weekly × 5 doses | 200 mg weekly × 2 doses | 1,000 mg × 1 dose | 1,000 - 2,000 mg, 1 - 2 doses | 100 mg × 3 doses | 1,000 mg × 1 dose | |
| Treatment duration | 4 weeks (once per week plus 1 dose 48 h pre-op) | 2 weeks (10 days, 1 day) | 1 day pre-op | 3 - 8 weeks | 1 week | 1 day pre-op | |
| EPO | Yesb | Yesc | No | No | No | Yes | |
| Comparator | Historic observation | Placebo | Placebo | PO Fe fumarate (200 mg) BID | PO iron fumarate (105 mg) or placebo | Placebo | |
| Outcomes | Blood | Hb measured baseline, at the start of surgery, initiation of CPB, every 15 min while on CPB, and at the end of VR | Hb 1 day before and within 24 h of hospital discharge | Hb, ferritin, iron, TSAT, reticulocyte, % anemic | Hb, iron, ferritin, transferrin, C-reactive protein, total iron binding capacity, EPO, achieve > 1.5 g/dL measured at least 3 weeks prior to surgery and again on the day of surgery | Hb, IRF, MCV, ferritin | Hb, reticulocyte, C-reactive protein, calculated RBC loss |
| Transfusion | Units per patient, number of patients transfused | Units transfused, number of patients transfused within 30 days | Units transfused, number of patients transfused | Units transfused, number of patients transfused | Units/patient, number of patients transfused | Number of units transfused | |
| Safety | Yes | Yes | Yes | Yes | Yes | Yes | |
| Mortality | Yes | Yes (30 day) | Yes | Yes | No | Yes | |
| Other | LOS, HF, CVA, MI, ARF, tamponade, re-op, infection, prolonged ventilation, thrombosis or failure of valve, endocarditis | LOS, peak troponin and CKMB, rates of MI, stroke, AKI, need for HD, and new onset AF | Safety (adverse events, vital signs, ECG, s-phosphate and hematology and biochemistry parameters) | LOS, QOL, AKI, AF, infection | LOS | LOS, infection, CVA, MI, AF, thrombosis, product acquisition costs, duration of mechanical ventilation | |
| Author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Quality |
|---|---|---|---|---|---|---|---|---|
| Urena et al, 2017 [34] | Low | Low | Low | Low | Low | Unclear | Low | Good |
| Johansson et al, 2015 [36] | Low | Low | Low | Low | Low | Unclear | Low | Good |
| Padmanabhan et al, 2018 [35] | Low | Low | High | High | Low | Unclear | High | Poor |
| Garrido-Martin et al, 2012 [33] | Low | Low | Low | Unclear | High | Unclear | Low | Good |
| Spahn et al, 2019 [37] | Low | Low | Low | Low | Low | Unclear | Low | Good |
| Author | Selection | Comparability | Outcome | Total | Quality |
|---|---|---|---|---|---|
| Cladellas et al, 2012 [32] | 3 | 1 | 2 | 6 | Fair |
| Author | Hb | Transfusion rate | Significant AE | QOL | Infections | LOS | Mortality |
|---|---|---|---|---|---|---|---|
| aOxford Centre for Evidence-based Medicine’s “Levels of Evidence.” https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/; bCincinnati Children’s Hospital Medical Center Evidence Collaboration’s, file:///Users/kellytankard/Downloads/Guideline%20Development%20Manual%20(1).pdf. AE: adverse event; Hb: hemoglobin; QOL: quality of life; LOS: length of stay. | |||||||
| Cladellas et al, 2012 [32] | X | X | X | X | X | X | |
| Urena et al, 2017 [34] | X | X | X | X | X | X | |
| Johansson et al, 2015 [36] | X | X | X | X | X | ||
| Padmanabhan et al, 2018 [35] | X | X | X | X | X | X | X |
| Garrido-Martin et al, 2012 [33] | X | X | X | X | |||
| Spahn et al, 2019 [37] | X | X | X | X | X | X | |
| Summary of Level of Evidencea | (6) 1b | (6) 1b | (6) 1b | (1) 2b | (6) 1b | (4) 1b | (5) 1b |
| Quality of Body of Evidenceb | Moderate | Moderate | Moderate | Low | Moderate | Moderate | Moderate |
| Author | Iron | EPO | Hb | Other significant hematologic findings | |||
|---|---|---|---|---|---|---|---|
| Baseline | Pre-op | Post-op | Follow-up | ||||
| *P < 0.05 between groups; **P < 0.05 from baseline; ***P < 0.001 between groups; aSD not reported. EPO: erythropoietin; Hb: hemoglobin; IV: intravenous; pre-op: preoperatively; POD: postoperative day; post-op: post-operatively; TSAT: transferrin saturation; SD: standard deviation. | |||||||
| Cladellas et al, 2012 [32] | IV | IV | 11.2 ± 1 | 12.6 ± 0.9* | - | - | |
| Historic observation | Historic observation | 10.9 ± 0.9 | 10.9 ± 0.9 | - | - | ||
| Urena et al, 2017 [34] | IV iron sucrose 200 mg at days 10 and 1 | Subcutaneous 0.75 µg/kg | 10.7 ± 1.2 | 10.9 ± 1.2* | 9.4 ± 1.6 (discharge) | - | No significant difference between pre-op, post-op, or discharge Hb after intervention |
| Placebo | Placebo | 11.3 ± 1.6 | 11.1 ± 1.2* | 9.9 ± 1.6 | - | ||
| Johansson et al, 2015 [36]a | IV | None | 14.3 | - | 10.2 (POD 5) | 12.4** (POD 28) | More non-anemic on follow-up |
| Placebo | 14 | - | 10.5 | 11.6** | |||
| Padmanabhan et al, 2018 [35] | IV | None | 8.9 | 9.8 | - | - | Increase in ferritin, decrease in EPO, TSAT |
| Oral | 11.1 | 12 | - | - | |||
| Garrido-Martin et al, 2012 [33] | IV | None | 14 ± 1.63 | 12.7 ± 1.64 | 11.1 ± 1.52 (POD 10) | 12.7 ± 1.40 (POD 30) | Higher ferritin |
| Oral | 13.7 ± 1.46 | 12.6 ± 1.70 | 11.0 ± 1.28 | 12.4 ± 1.27 | |||
| Placebo | 14 ± 1.35 | 12.8 ± 1.29 | 11.0 ± 1.44 | 12.3 ± 1.15 | |||
| Spahn et al, 2019 [37] | IV | Subcutaneous 40,000 units | 12.8 ± 1.5 | - | 9.2*** (POD 5) | 9.1*** (POD 7) | Higher reticulocyte count |
| Placebo | 12.9 ± 15 | - | 8.7*** | 8.5*** | |||
| Author | Intervention | Transfusion | ||
|---|---|---|---|---|
| Transfusion trigger | Transfusion rate, n/N (%) | Median number of units transfused | ||
| *P < 0.05 between groups; **P < 0.001 between groups. Hb: hemoglobin; IV: intravenous; IM: intramuscular; NR: not reported; EPO: Epogen; SC: subcutaneous; ICU: intensive care unit. | ||||
| Cladellas et al, 2012 [32] | IV iron + IV EPO | Hb < 7 g/dL | 50/75 (67) | NR |
| Historic observation | 55/59 (93)** | NR | ||
| Urena et al, 2017 [34] | IV iron + IM EPO | Hb < 7 g/dL, Hb 7 - 8 g/dL if symptomatic | 13/48 (27) | 1 |
| Placebo | 13/52 (27) | 2 | ||
| Johansson et al, 2015 [36] | IV | NR | 8/30 (27) | 1 |
| Placebo | 11/30 (37) | 2 | ||
| Padmanabhan et al, 2018 [35] | IV | NR | 16/20 (80) | 1.5 |
| Oral | 12/20 (60) | 2 | ||
| Garrido-Martin et al, 2012 [33] | IV | Hb < 8 g/dL in coronary patients, Hb < 7 g/dL in valve surgery patients | 20/54 (37) | 0 |
| Oral | 27/53 (51) | 1 | ||
| Placebo | 26/50 (50) | 0.5 | ||
| Spahn et al, 2019 [37] | IV + SC EPO | Hb < 7 - 8 g/dL intraoperative and ICU, Hb < 8 g/dL on wards | 108/243 (44) | 0 |
| Placebo | 127/241 (53)* | 1 | ||
| Author | Surgery | Significant AEa | QOL | Infections | LOS, median (IQR) | Mortality |
|---|---|---|---|---|---|---|
| aAs defined by study. AE: adverse event; CABG: coronary artery bypass grafting; diff: difference; IQR: interquartile range; LOS: length of stay; NR: not reported; QOL: quality of life; TAVI: trans-aortic valve implantation; VR: valve replacement. | ||||||
| Cladellas et al, 2012 [32] | VR | None | NR | Decreased: 8% vs. 24%, P = 0.01 | Shorter: 10 days (8 - 14) vs. 15 days (10 - 27), P < 0.01 | Decrease: 9% vs. 23%, P = 0.04 |
| Urena et al, 2017 [34] | VR (TAVI) | No diff | NR | No diff | No diff | No diff |
| Johansson et al, 2015 [36] | CABG, VR | No diff | NR | No diff | NR | No diff |
| Padmanabhan et al, 2018 [35] | CABG, VR | None | No diff | No diff | No diff | No diff |
| Garrido-Martin et al, 2012 [33] | CABG, VR | None | NR | Not increased | NR | NR |
| Spahn et al, 2019 [37] | CABG, VR | No diff | NR | No diff | No diff | No diff |