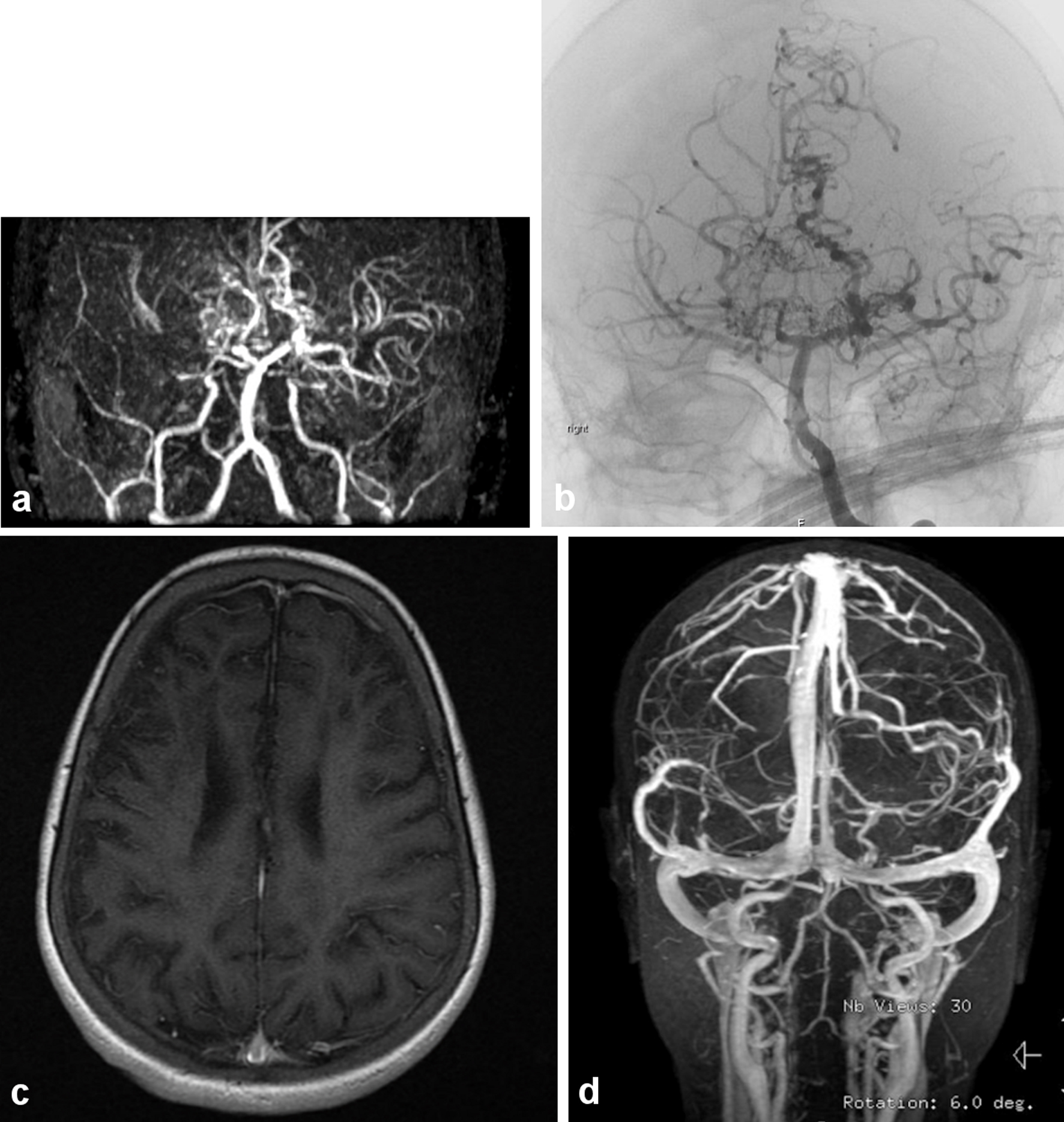
Figure 1. (a) The magnetic resonance (MR) angiography of the intracranial circulation showed significant prominent collateral vessels in the posterior cerebral artery territory with evidence for probable retrograde filling of middle cerebral artery territory from the posterior circulation. Significant collateral vessel prominence especially in the perforator branches in the thalamic region is detected. The findings are consistent with moyamoya disease with major thinning and attenuation in the supraclinoid internal carotid artery, and associated multiple watershed zone infarctions (before HSCT: March, 2009). (b) Evidence of narrowing at the distal internal carotid artery in the supraophthalmic segment bilaterally with the development of significant amount of small perforators giving puff of smoke appearance characteristic of moyamoya disease (same patient after HSCT in July, 2011). (c) Significant cortical and subcortical encephalomalacia diffusely along supratentorial brain parenchyma, significant within right frontal lobe with evidence of hemodynamic ischemic changes along the bilateral centrum semiovale. (d) Magnetic resonance imaging (MRI) with normal vascular distribution with moderately severe sickle cell disease.