| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 4, August 2022, pages 154-158
Durable Remission in Hodgkin Lymphoma Treated With One Cycle of Bleomycin, Vinblastine, Dacarbazine and Two Doses of Nivolumab and Brentuximab Vedotin
Binoy Yohannana, Adan Riosa, c, Maximilian Bujab
aDivision of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center, Houston, TX, USA
bDepartment of Pathology and Laboratory Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, USA
cCorresponding Author: Adan Rios, Division of Hematology/Oncology, McGovern Medical School, The University of Texas Health Science Center, Houston, TX, USA
Manuscript submitted July 17, 2022, accepted August 8, 2022, published online August 30, 2022
Short title: Durable Remission in HL After Nivolumab and BV
doi: https://doi.org/10.14740/jh1035
| Abstract | ▴Top |
A 49-year-old woman with systemic lupus erythematosus, lupus nephritis and chronic congestive heart failure presenting with “bulky” cervical lymphadenopathy was diagnosed with classic Hodgkin lymphoma (HL) stage IIIB (positron emission tomography-computed tomography (PET-CT) scan and bone marrow biopsy). She received one cycle of bleomycin, dacarbazine, and vinblastine to debulk the tumor. Given her advanced heart failure, doxorubicin was not administered. After the first cycle of chemotherapy, she was switched to nivolumab plus brentuximab vedotin (BV) and received two doses 4 weeks apart, finishing in July 2019. A restaging PET-CT in June 2019 showed a complete remission (CR). After the second course of treatment, she was unable to tolerate more treatments and hence was placed on a surveillance program. She remains in CR after a follow-up of 3 years. This case highlights the role of a tailored treatment approach to optimize clinical outcomes in uniquely complex clinical circumstances. BV in combination with nivolumab is a reasonable alternative regimen in HL ineligible for cytotoxic chemotherapy.
Keywords: Hodgkin lymphoma; Autoimmune disease; Durable remission; Ineligible for chemotherapy; Brentuximab vedotin; Nivolumab
| Introduction | ▴Top |
Hodgkin lymphoma (HL) was first reported by Thomas Hodgkin in 1832. It accounts for 10% of all lymphomas [1]. HL has a bimodal age distribution with the initial peak seen in young adults and the second in older patients. Patients with autoimmune diseases are at an increased risk for Hodgkin and non-Hodgkin lymphomas (NHL) [2-4]. HL is considered curable in most patients; however, in patients with medical comorbidities that prohibit cytotoxic chemotherapy, clinical outcomes are inferior. There is an unmet need for novel therapies in this population. We report a 49-year-old female patient with HL and multiple medical comorbidities who achieved a durable remission after successful treatment with an attenuated chemoimmunotherapy regimen.
| Case Report | ▴Top |
Investigations
A 49-year-old woman with morbid obesity (body mass index (BMI) > 40), systemic lupus erythematosus (SLE), lupus nephritis, diabetes, coronary artery disease with chronic congestive heart failure (CHF), atrial fibrillation (AF), and multiple strokes with residual left-sided weakness presented with a 3-week history of fever, chills, night sweats, 20 lb weight loss, and bilaterally swollen neck lymph nodes. Computed tomography (CT) of the neck showed bulky cervical lymphadenopathy (Fig. 1). CT scan of the chest showed bulky right supraclavicular, infraclavicular, mediastinal, right hilar, and right axillary adenopathy. CT abdomen showed enlarged sub-centimeter retroperitoneal lymph nodes with largest measuring 9.3 mm in the aortocaval region. The transthoracic echocardiogram showed an ejection fraction < 20%. Cardiac magnetic resonance imaging showed a left ventricular ejection fraction of 16% and right ventricular ejection fraction of 17%. She denied hemoptysis, recent travel, or sick contacts. Other comorbidities included chronic obstructive pulmonary disease, history of pulmonary embolism (PE), peripheral vascular disease, hypertension, and hyperlipidemia. The patient was a non-smoker. The Charlson comorbidity index score was 12 with an ECOG performance status of 4. She had a sister with SLE and breast cancer. The patient was initially taking apixaban for deep vein thrombosis but later was switched to warfarin for recurrent venous thromboembolism. Workup for antiphospholipid antibody syndrome was negative. She was taking hydroxychloroquine 200 mg twice daily, leflunomide 10 mg daily, and prednisone 5 mg daily for SLE. Her other medications included clopidogrel, metoprolol, ivabradine, sacubitril-valsartan, allopurinol, empagliflozin, atorvastatin, and escitalopram. Physical examination showed bilaterally swollen neck lymph nodes with a 4 × 5 cm conglomerate of matted nodes in the right side of the neck. She did not have hepatosplenomegaly.
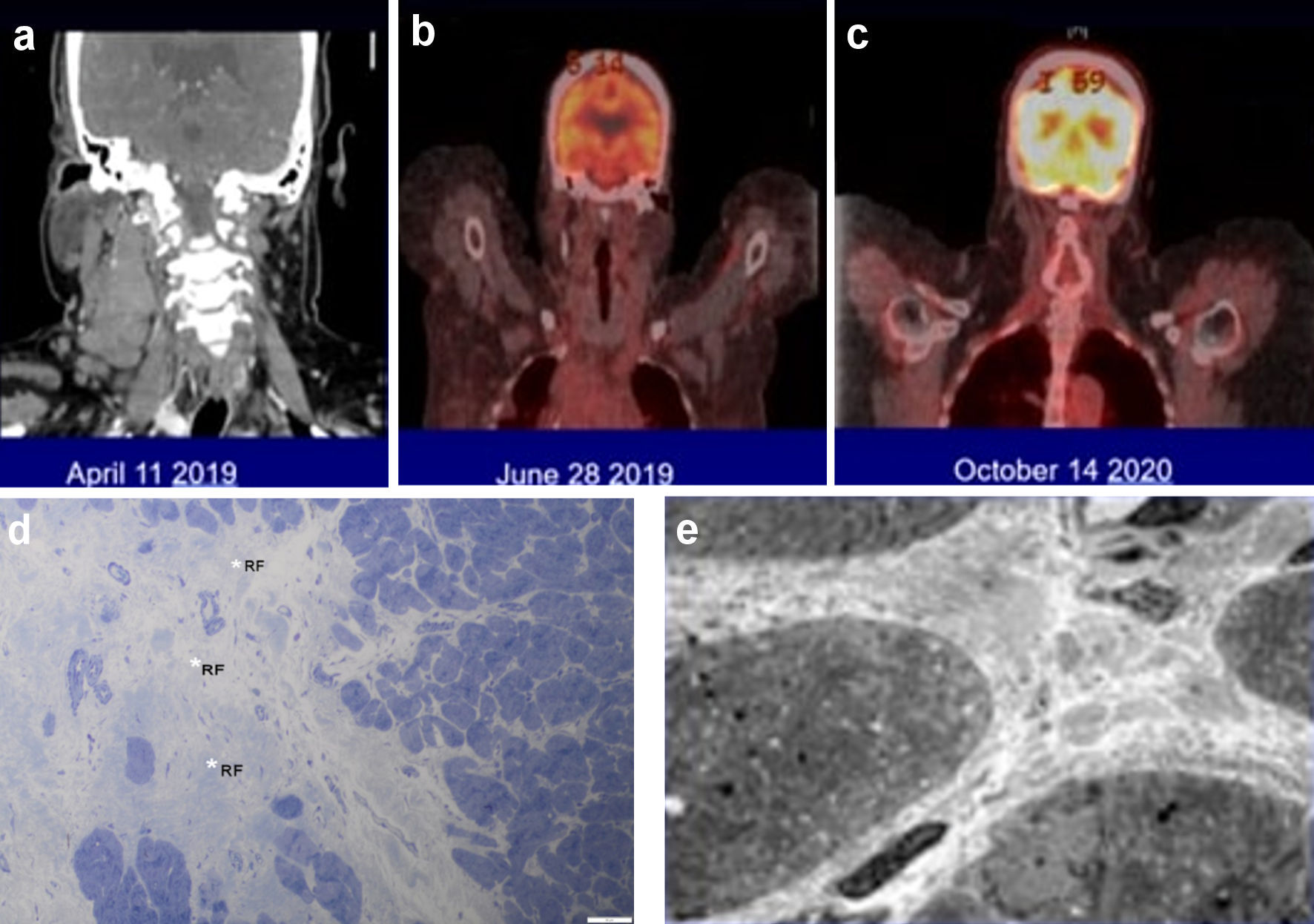 Click for large image | Figure 1. (a-c) The large neck lymph nodes involved by Hodgkin lymphoma and the dramatic response to the combination of an immune checkpoint inhibitor with brentuximab vedotin. (d, e) Endomyocardial biopsy findings by light (d) and electron (e) microscopy. (d) The myocardium is free of active inflammatory infiltrates but exhibits evidence of previous myocardial insult represented by a focus of replacement fibrosis (RF, white asterisk) (1-µm section stained with toluidine blue, × 500). (e) Some areas of myocardium had increased interstitial collagen with an occasional macrophage but no aggregates of inflammatory cells between the cardiomyocytes (× 2,000). |
Diagnosis
A cervical lymph node biopsy showed an effaced nodal architecture with lymphoid infiltrate and extensive necrosis. Tumor cells were scattered, with focal clusters of large mononuclear and multinuclear large cells. The inflammatory background had small lymphocytes, histiocytes, plasma cells, and scattered eosinophils. Immunohistochemical stains were positive for CD15 (weak), CD30, BCL6, PAX5, and MUM1 and negative for CD3, CD4, CD5, CD7, CD8, CD45, CD79a, and ALK. Tumor cells were positive for EBER1. These findings were consistent with classic Hodgkin lymphoma, mixed cellularity subtype. A bone marrow biopsy was negative for HL. A positron emission tomography (PET-CT) confirmed a clinical stage IIIB HL.
Treatment
The patient received one cycle of bleomycin, dacarbazine, and vinblastine to debulk the tumor. Given her medical comorbidities, doxorubicin was not administered. At the time when we encountered this case (2019), Herrera et al had published the interim results of a phase 1/2 study of nivolumab in combination with brentuximab vedotin (BV) showing impressive response rates (objective response rate (ORR) = 81%, complete response (CR) = 61%) in patients with relapsed or refractory HL and avoiding the usual toxicities associated with chemotherapy [5]. We felt this would be a good chemotherapy-free regimen for our patient. We provided our patient with all the pertinent information regarding this regimen, including the risk of immune-related adverse events and potential for exacerbation of SLE with the use of nivolumab. After carefully reviewing the potential risks versus benefit, our patient elected to proceed with the chemotherapy-free regimen. She was switched to nivolumab (240 mg) plus BV (1.8 mg/kg) and received two doses of this regimen 4 weeks apart, finishing in July 2019. A restaging PET-CT in June 2019 showed a complete remission (CR, Deauville Criteria 1).
Follow-up and outcomes
After the second course of treatment, she had a PE with CHF exacerbation and elevated troponin that peaked at 1.97 µg/L (reference range: ≤ 0.04 µg/L). This prompted a cardiac catheterization with an endomyocardial biopsy (EMB). The EMB showed no inflammatory infiltrate or cardiomyocyte necrosis, ruling out an immune checkpoint inhibitor (ICI; nivolumab)-associated myocarditis (Fig. 1). She was unable to tolerate more treatments. A PET scan done 2 months later showed CR (Fig. 1). Acknowledging her multiple medical comorbidities and her CR from HL, we placed her on a surveillance program. Clinical and restaging PET-CT scans done at 6-month intervals have continued to show CR with 3 years of follow-up.
| Discussion | ▴Top |
HL is a highly curable B cell lymphoproliferative disorder even in advanced stages of the disease. Six to eight cycles of an anthracycline-based chemotherapy regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is considered the standard of care in newly diagnosed HL [6]. In patients with stage III and IV HL, BV in combination with doxorubicin, vinblastine, and dacarbazine (AVD) has shown excellent results with a superior progression free survival (PFS) and overall survival (OS) when compared to ABVD alone [7]. Although HL is considered highly curable, cytotoxic chemotherapy regimens can be extremely challenging to administer in frail and elderly patients with comorbidities. Moreover, these unfit patients with comorbidities are often excluded from prospective clinical trials. Elderly patients tend to have inferior outcomes due to intolerance to standard chemotherapy and high treatment-related mortality (TRM). The North American intergroup trial (E2496) investigated the ABVD regimen in older HL patients and noted significant bleomycin pulmonary toxicity (24%) and relatively high TRM (9%) [8]. Similarly, a TRM of 5% was noted in the phase 3 ECHELON-1 study of older patients with HL and all the deaths were related to pulmonary toxicity [9]. An anthracycline-free regimen may be better tolerated; however, it can compromise the curative potential [10]. There is a paucity of data on optimum treatment approach in HL patients who are ineligible for cytotoxic chemotherapy.
BV is an antibody drug conjugate targeting CD 30 that has shown promising single-agent activity in relapsed/refractory HL [11, 12]. Also, frontline BV monotherapy in elderly patients has yielded outstanding results with an ORR of 92% and median OS of 77.5 months [13]. Friedberg et al reported a phase 2 nonrandomized study of BV plus dacarbazine in newly diagnosed classical HL patients older than 60 years (n = 22) ineligible for cytotoxic chemotherapy. BV and dacarbazine was well tolerated with an ORR and CR rate of 100% and 62%, respectively. The median PFS was 17.9 months [14]. Sequential BV and AVD was investigated in a phase 2 trial of older patients with HL. Patients received two initial doses of single-agent BV, followed by six cycles of AVD and then BV consolidation. The ORR was 82% and CR rate was 36% after the first two doses of BV and it improved to 95% and 90%, respectively, after six cycles of AVD. Patients who had a favorable response (CR/PR) to the first two doses of BV had a significantly better outcome than non-responders. Patients with a higher Cumulative Illness Rating Scale-Geriatric (CIRS-G) comorbidity score (≥ 10) had significant worse 2-year PFS rate of 45% compared to 100% for those with a lower CIRS-G score (< 10). Although 42% of patients experienced grade 3 or 4 adverse event, the TRM was only 2% [15].
Patients with HL have overexpression of PD-L1 and PD-L2 on the surface of Reed-Sternberg cells due to genomic amplification of the 9p24.1 locus [16]. Also, mutations in beta-2 microglobulin are common in HL leading to T cell dysfunction [17]. All these factors make immunotherapy an exciting therapeutic option in HL. Single-agent nivolumab has shown an impressive ORR of 66% in patients who have progressed after an autologous stem cell transplant (ASCT) and BV [18]. Nivolumab in combination with BV is a highly effective salvage regimen in relapsed HL. A phase 2 study (n = 91) investigated BV and nivolumab in the second-line setting, and results were quite encouraging, with an ORR of 85% and CR of 67%. The 3-year PFS was 91% in patients who received an ASCT [19]. In the front-line setting, the combination of BV and nivolumab has been investigated in two small studies. In a phase 2 study of 20 elderly patients with HL, a combination of BV and nivolumab achieved an impressive ORR of 95%, and the median OS was not reached. No TRM was reported [20]. In the Academic and Community Cancer Research United (ACCRU) single-arm phase 2 study, 46 elderly patients with newly diagnosed HL ineligible for chemotherapy were treated with eight cycles of BV-nivolumab, and this regimen showed an ORR of 61% with 48% complete metabolic response [21]. Similarly, single-agent pembrolizumab has shown durable remissions with a CR rate of 28-32% in heavily pretreated (≥ 3 prior lines of therapy) patients with classic HL, and the median time to response was 2.8 months [22].
Epstein-Barr virus (EBV) accounts for 30-50% cases of HL, and EBV-associated HL tends to have a poor outcome [23]. Our patient had EBV-positive HL, and her numerous medical comorbidities added to the complexity of the case. We had to come up with a balanced treatment approach to achieve remission and to minimize treatment-related toxicity. The use of ICIs can increase the risk of SLE exacerbation, and this was a matter of concern in our patient. Given the curative potential of ICIs, there is growing evidence to suggest that they can be safely used in patients with autoimmunity, provided there is significant immunotherapy expertise [24]. After carefully reviewing the potential risks and benefits, we decided to treat her with an ICI despite her history of SLE and lupus nephritis. Our patient received two doses of nivolumab and BV, tolerating this regimen well without exacerbation of her underlying autoimmune disease. Although the upfront use of BV in combination with nivolumab in this fashion has not received regulatory approval by North American or European regulatory agencies, its use is endorsed by the National Comprehensive Cancer Network (NCCN) guidelines in the second-line setting [25]. In conclusion, HL treatment for unfit patients should be tailored considering frailty and medical comorbidities. A combination of BV plus nivolumab is well tolerated and is a reasonable alternative regimen for HL patients ineligible for cytotoxic chemotherapy.
Learning points
The optimal treatment approach in HL patients who are ineligible for chemotherapy is unknown and should be tailored based on comorbidities and performance status.
BV in combination with nivolumab is a reasonable alternative regimen for patients with HL ineligible for cytotoxic chemotherapy.
Autoimmune diseases are not absolute contraindications for ICI therapy. The treating physician should make a shared decision after carefully reviewing the risks versus benefits with the patient.
Acknowledgments
The authors would like to thank Ms. Virginia Mohlere for editorial assistance.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
BY contributed to concept, design, data collection and initial draft of the manuscript. AR selected the treatment regimen, treated the patient, and revised the manuscript for important intellectual content. MB provided pathology expertise in analyzing the case and important intellectual content.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Hodgkin. On some morbid appearances of the absorbent glands and spleen. Med Chir Trans. 1832;17:68-114.
doi pubmed - Bernatsky S, Ramsey-Goldman R, Isenberg D, Rahman A, Dooley MA, Sibley J, Boivin JF, et al. Hodgkin's lymphoma in systemic lupus erythematosus. Rheumatology (Oxford). 2007;46(5):830-832.
doi pubmed - Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337-2344.
doi pubmed - Westermann R, Zobbe K, Cordtz R, Haugaard JH, Dreyer L. Increased cancer risk in patients with cutaneous lupus erythematosus and systemic lupus erythematosus compared with the general population: A Danish nationwide cohort study. Lupus. 2021;30(5):752-761.
doi pubmed - Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183-1194.
doi pubmed - Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, Green MR, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478-1484.
doi pubmed - Straus DJ, Dlugosz-Danecka M, Connors JM, Alekseev S, Illes A, Picardi M, Lech-Maranda E, et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021;8(6):e410-e421.
doi - Evens AM, Hong F, Gordon LI, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161(1):76-86.
doi pubmed - Evens AM, Connors JM, Younes A, Ansell SM, Kim WS, Radford J, et al. Older patients (pts) with previously untreated classical Hodgkin lymphoma (cHL): A detailed analysis from the phase 3 ECHELON-1 study. Blood. 2018;132(Supplement 1):1618-1618.
doi - Sykorova A, Mocikova H, Lukasova M, Koren J, Stepankova P, Prochazka V, et al. Outcome of elderly patients with classical Hodgkin's lymphoma. Leuk Res. 2020;90:106311.
doi pubmed - Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236-1243.
doi pubmed - Zinzani PL, Viviani S, Anastasia A, Vitolo U, Luminari S, Zaja F, Corradini P, et al. Brentuximab vedotin in relapsed/refractory Hodgkin's lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica. 2013;98(8):1232-1236.
doi pubmed - Yasenchak CA, Bordoni RE, Patel-Donnelly D, Larson T, Goldschmidt JH, Boccia R v., et al. Frontline brentuximab vedotin as monotherapy or in combination for older Hodgkin lymphoma patients. J Clin Oncol. 2020;38(15_suppl):8032-8032.
doi - Friedberg JW, Forero-Torres A, Bordoni RE, Cline VJM, Patel Donnelly D, Flynn PJ, Olsen G, et al. Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged ≥60 years with HL. Blood. 2017;130(26):2829-2837.
doi pubmed - Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovanovic BD, Bociek GR, et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol. 2018;36(30):3015-3022.
doi pubmed - Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3277.
doi pubmed - Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101(7):794-802.
doi pubmed - Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283-1294.
doi - Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138(6):427-438.
doi pubmed - Yasenchak CA, Bordoni R, Yazbeck V, Patel-Donnelly D, Anderson T, Larson T, et al. Phase 2 study of frontline brentuximab vedotin plus nivolumab in patients with Hodgkin lymphoma aged ≥60 years. Blood. 2019;134(Supplement_1):237-237.
doi - Cheson BD, Bartlett NL, LaPlant B, Lee HJ, Advani RJ, Christian B, Diefenbach CS, et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2020;7(11):e808-e815.
doi - Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin LYMPHOMA. J CLIN ONCOL. 2017;35(19):2125-2132.
doi pubmed - LIU TY, Wu SJ, Huang MH, Lo FY, Tsai MH, Tsai CH, Hsu SM, et al. EBV-positive Hodgkin lymphoma is associated with suppression of p21cip1/waf1 and a worse prognosis. Mol Cancer. 2010;9:32.
doi pubmed - Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, Larkin J, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020;31(6):724-744.
doi pubmed - Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, Benitez CM, et al. Hodgkin Lymphoma, Version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(6):755-781.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


