| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Letter to the Editor
Volume 11, Number 6, December 2022, pages 240-245
Unveiling the Great Therapeutic Potential of MASPs as Hemostatic Agents
Unit of Pediatric Hematologic Oncology and BMT, Sultan Qaboos University Hospital, Muscat, Oman
Manuscript submitted September 22, 2022, accepted October 31, 2022, published online December 1, 2022
Short title: Potention of MASPs as Hemostatic Agents
doi: https://doi.org/10.14740/jh1060
| To the Editor | ▴Top |
The lectin complement pathway (LP) is an important effector arm of innate immunity and exemplary pattern recognition artist that draws a fine line between friend and foe (host defense) and between innocuous and noxious (homeostasis). Intriguingly, the proteolytic activity of the LP is attributed to proteolytic enzymes, called mannan-binding lectin (MBL)-associated serine proteases (MASPs). MASPs are central components of the LP that resemble the serine proteases, C1r and C1s, of the classical complement pathway (CP). Recently, the MASPs’ important role in the coagulation cascade was unmasked by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1-6]. MASPs (mainly MASP-1) are actually key elements that connect both complement and coagulation systems [7]. So far, research into complement-coagulation interactions focusing merely on MASP inhibition had offered promising targets for novel preventive and therapeutic strategies [8]. Conversely, here I propose that the fibrinolytic activity of MASPs can be explored in the management of bleeding disorders. In particular, traumatic and surgical bleeding are life-threating but potentially avoidable causes of death [9, 10]. Johnston et al showed that postsurgical bleeding is associated with substantial increases in postprocedural length of stay, days spent in critical care, and the risks of infection, vascular events, acute renal failure, and in-hospital mortality [11]. In addition, use of anticoagulants and antiplatelets increases the surgical bleeding risk, creates a need for multiple pharmacologic approaches and poses potential problems in managing surgical patients [12-15]. On the other hand, approximately one-third of all trauma patients with bleeding present with a coagulopathy on hospital admission [16, 17]. This subset of patients has a significantly increased incidence of multiple organ failure and death compared to patients with similar injury patterns in the absence of a coagulopathy [18]. Coagulopathy frequently occurs early in the postinjury period and is an independent predictor of mortality. Compared to patients whose initial prothrombin time (PT) and activated partial thromboplastin time (aPTT) are normal, trauma patients have 35% and 326% increased risk of mortality when their initial PT and aPTT are abnormal, respectively [19]. It is important to emphasize that trauma-induced coagulopathy, also called acute traumatic coagulopathy, is distinct from massive transfusion coagulopathy that occurs in the context of loss and dilution coagulopathy [20, 21] or disseminated intravascular coagulation [22].
Hitherto, measures to reduce intraoperative blood loss have been limited to enhancement of coagulation by recombinant activated coagulation factor VII (rFVIIa), desmopressin, fibrinogen, prothrombin complex concentrates, inhibition of fibrinolysis comprising lysine analogues (tranexamic acid and epsilon aminocaproic acid) and a broad-spectrum serine protease inhibitor (aprotinin) in order to avoid or minimize the need for blood transfusions, which is directly proportional to perioperative complications and mortality [23]. Moreover, since FXa is at a critical point of the coagulation cascade (FXa is the trypsin-like proteinase of coagulation that catalyses prothrombin activation) [24], several pharmacological strategies have been developed to modulate its function [25]. These endeavours paved the way for a new era of non-vitamin K oral anticoagulants that not only produce more predictable/less labile anticoagulant effect than oral vitamin K antagonists (such as warfarin), but are also equally safe and effective [26]. These non-vitamin K oral anticoagulants have been termed direct oral anticoagulants that include the factor Xa inhibitors (i.e., rivaroxaban, apixaban, edoxaban, and betrixaban) and direct thrombin inhibitors (i.e., dabigatran) [27]. Contrarily, harnessing FXa for its procoagulant effect in the context of prothrombin activation can prove challenging due to two main factors. Firstly, the ability of Xa to activate prothrombin (FII) is markedly low without its activated cofactor, FVa. Actually, the catalytic efficiency of FXa activation of prothrombin increases by 100,000-fold when FXa incorporates into the prothrombinase complex, a composition of phospholipids, Ca2+, FVa cofactor and FXa, which cleaves prothrombin at Arg271 and Arg320 [28]. To put it simply, the prothrombinase complex is essential for hemostasis as it is the only physiologic producer of thrombin [29]. Secondly, despite its negligible contribution to the catalytic process, the membrane surface is obligately required. Normally, the prothrombinase complex is physiologically assembled on phospholipid membranes at the site of tissue damage; the most relevant of which is the activated platelet surface [30]. The membrane surface provides an environment in which both the FVa/FXa complex and prothrombin (the prothrombinase substrate) can co-concentrate. Activated platelets also provide FVa, the essential nonenzymatic cofactor of the prothrombinase complex. Without FVa, FXa will not bind to activated platelets [31]. Despite that only 18% to 25% of the total FV in blood is stored as a mixture of FV and FVa within the α-granules of platelets, platelet-derived FV play a more important role in hemostasis than its plasma counterpart [32]. In other terms, both platelets and FVa are required for explosive thrombin generation. Although prothrombin cleavage by prothrombinase is one of the most extensively studied reactions in the process of blood clotting, the mechanistic details regarding prothrombin activation are replete with challenges and controversies [33].
Interestingly, the complement and the coagulation systems are two sides of the same coin. Since they have been derived from the same ancestral pathways, extensive and reciprocal molecular cross-talks between them have been discovered [34-36]. Both proteolytic cascades are composed of serine proteases with common structural characteristics and their interactions form a complex serine protease network [37]. To further elaborate on the potential crosstalk between complement and coagulation pathways, it is important to appreciate substitute routes of complement activation via the “extrinsic protease” pathway, where proteases such as plasmin, thrombin, and plasma kallikrein can cleave and activate C3 [38]. Reciprocally, components of the complement system potentiate coagulation and inhibit fibrinolysis, mainly through C5a, in order to additionally provide a mechanical barrier against the spread of invading bacteria [39]. However, the proteolytic conversion of prothrombin to thrombin is regarded as the most critical step in the coagulation cascade (thrombin mediates the functions that leads to the formation of blood clots by cleavage of fibrinogen and FXIII as well as activation of platelets) [40]. Despite being considered to be of common origin, the proteases C1r and C1s of the CP do not cleave prothrombin but those of the LP do. The immune system deploys LP as a scavenger system that is activated through sequential enzymatic reactions when pattern-recognition molecules (PRMs) bind to damage-associated molecular patterns (DAMPs) and/or pathogen-associated molecular patterns (PAMPs). PRMs constitute two distinct major lectin groups: collectins and ficolins (Table 1). Neither collectins nor ficolins possess enzyme activity themselves but rely on MASPs (MASP-1, MASP-2, and MASP-3), with which they circulate in complexes [41]. Binding of LP-recognition complexes (MBL/MASP complexes) to carbohydrate and acetylated residues on the surface of pathogens and altered (apoptotic, necrotic, malignant, or damaged) host cells converts MASP zymogens into their enzymatically active form that drive LP-mediated complement activation. Out of the three MASPs, MASP-3 is not wandering beyond the complement system and has no clear role in the coagulation cascade. As the exclusive pro-factor D (FD) activator in resting blood, MASP-3 plays a pivotal role in the alternative complement pathway (AP), and thus, considered a fundamental link between the lectin and alternative pathways [42]. In contrast, MASP-1 and MASP-2 are akin to the coagulation proteases in their catalytic properties of activating fibrinogen and prothrombin respectively (Fig. 1). To further elaborate, MASP-1 has thrombin-like activity whereby it cleaves and activates fibrinogen and FXIII; whereas MASP-2 has FXa-like activity whereby it activates prothrombin through cleavage to form thrombin [43].
 Click to view | Table 1. Pattern-Recognition Molecules of the Lectin Pathway |
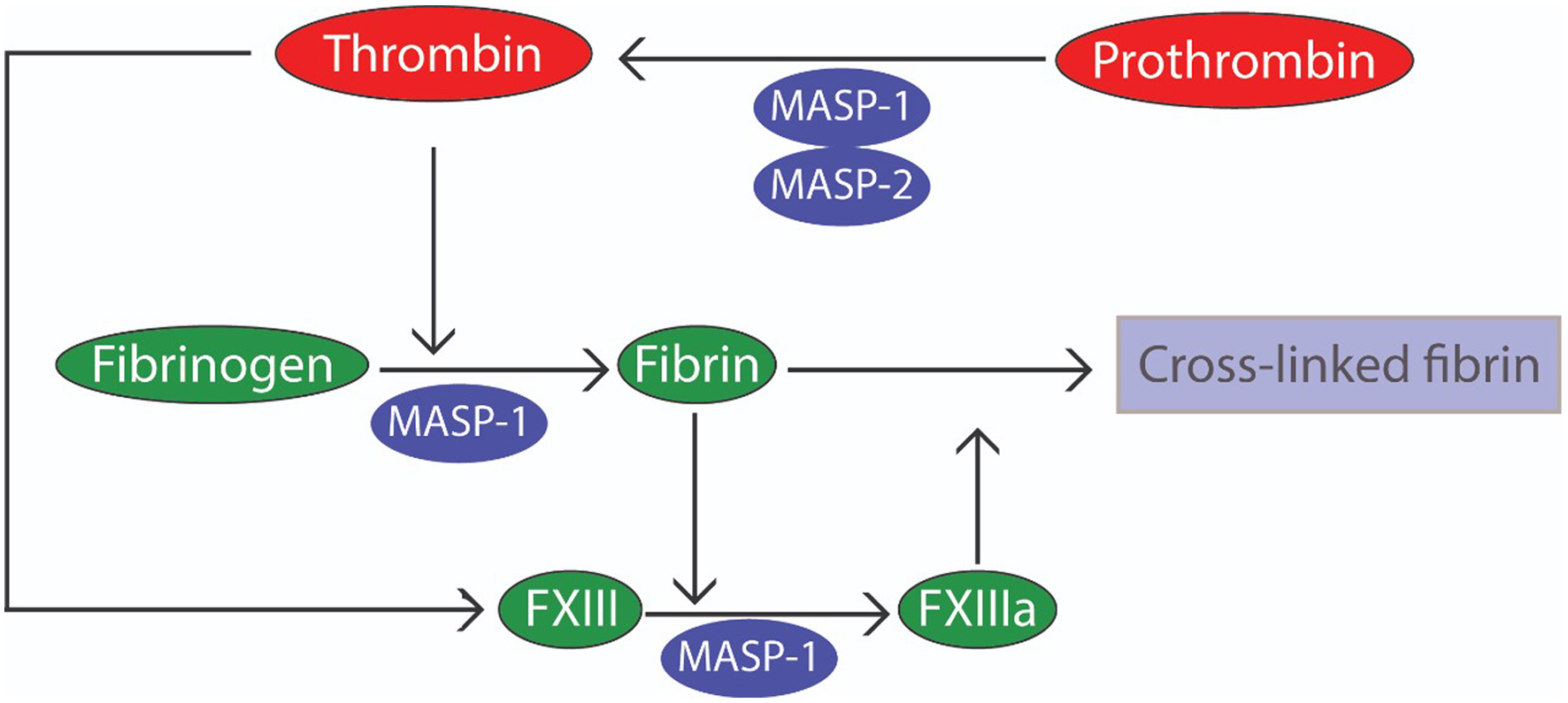 Click for large image | Figure 1. The coagulation cascade is composed of clotting factors (serine protease zymogens) and their cofactors. Effective hemostasis relies on the timely production of thrombin via prothrombinase, a Ca2+-dependent complex of factor Va (FVa) and FXa assembled on the activated platelet surface, which cleaves prothrombin (FII) at Arg271 and Arg320. In contrast, MASP-1 cleaves prothrombin at Arg393 and directly cleaves fibrinogen and activates FXIII due to its thrombin-like activity. On the other hand, MASP-2 is capable of activating prothrombin in a similar manner to FXa which indirectly cleaves both FXIII and fibrinogen through the active thrombin generated from prothrombin. FXIII and fibrinogen are unusual among clotting factors as neither is a serine protease. Fibrin acts as both the substrate and cofactor for FXIII; and once activated, the transglutaminase FXIIIa is capable of stabilizing fibrin clots by ligating adjacent fibrin monomers. MASP: mannan-binding lectin-associated serine protease. |
MASP-2 is the LP-effector enzyme and a hallmark of profound activation of the LP [44]. Only MASP-2 can cleave both C2 and C4 in order to generate C3 convertase that mediates downstream activation of the complement system. MASP-2 also promotes clotting by prothrombin cleavage similar to FXa-mediated cleavage [45] and by cleavage of FXII to FXIIa [46]. In order to establish the concept of using MASP-2 as a procoagulant, it is important to appreciate the effect of its inhibition. Within this context, MASP-2 inhibition has garnered special attention especially that this would spare the immune defence ability of the CP [47]. In particular, MASP-2 has been incriminated in the development of three major thrombotic microangiopathies (TMAs) [48], thrombotic thrombocytopenic purpura (TTP), atypical hemolytic uremic syndrome (aHUS) and “secondary” aHUS-type TMAs, occurring in the setting of infections (e.g., severe coronavirus disease 2019 (COVID-19) [49]), autoimmune disease or transplant-associated TMA (TA-TMA) [50, 51]. Not surprisingly, the MASP-2 inhibitor narsoplimab, a fully humanized immunoglobulin gamma 4 (IgG4) monoclonal antibody against MASP-2, showed efficacy and safety in the treatment of adult patients with severe HSCT-TMA [52, 53] and COVID-19 patients with acute respiratory distress syndrome (ARDS) [54]. Krarup et al [55] have demonstrated that MASP2-mediated activation of prothrombin is relatively specific as MASP2 homologues (C1r and C1s) do not activate prothrombin, and MASP2 does not activate other protease proenzymes. Their results showed that the thrombin activation potential is quite low (about 4-5% the rate observed with FXa) and presumably much lower than would be observed for the prothrombinase complex. Moreover, MASP2 will only activate prothrombin near any surface where MBL or the ficolins can bind [55]. A recent study has revealed that lectin-MASP-2 conjugates have a great potential to be used as anti-microbial and anti-cancer agents [56]. Knowing that lectins are carbohydrate-binding proteins and in order to apply this theory in bleeding disorders we need to search for glycan epitopes with which lectin-MASP-2 conjugates can bind at the bleeding vessel. Following vascular injury, coagulation is initiated by the exposure of subendothelial tissue factor (TF) on extravascular cells, which then interacts with FVIIa to activate FX [57]. TF is a glycosylated transmembrane protein (also called FIII, tissue thromboplastin or CD142) that undergoes post-translational modification by N-linked glycosylation. N-linked glycans on human glycoproteins like TF are attached to the amide nitrogens of asparagine (Asn) side chains. TF has N-linked glycosylation consensus sequences at three positions (Asn-11, Asn-124, and Asn-137) [58]. Consequently, synthesizing lectin-MASP-2 conjugates containing synthetic lectins that specifically bind these N-linked glycosylation sequences of TF would theoretically result in targeted activation of the coagulation cascade at the bleeding sites. Notably, the natural MASP-2 can be substituted by the highly potent recombinant MASP-2 [59].
Being the most abundant MASP in the complement system, MASP-1 first autoativates and then transactivates MASP-2 followed by generating the C3 convertase (C4b2a) by cleaving C2 and C4 (C2 by MASP-1 and C2 and C4 by MASP-2) upon binding of MBL/MASP complexes to their cognate ligands. Ostensibly an essential central component of the LP, MASP-1 also acts like thrombin, a key protease of the coagulation process, as it cleaves fibrinogen and FXIII by possessing similar Arg selectivity, and is thus, able to catalyse the formation of cross-linked fibrin [60]. MASP-1 is a promiscuous and relatively potent protease with broad substrate specificity that resembles that of trypsin rather than MASP-2 [61]. The ancient origin of MASP-1 and its thrombin-like activity suggests its involvement in a coagulation-based defence mechanism in the early evolution of innate immunity [62]. Hess et al [63], by using a recombinant mutant catalytic fragment of MASP-1, showed that MASP-1 directly activates prothrombin, as well as three natural substrates for thrombin in plasma (fibrinogen, FXIII and thrombin-activatable fibrinolysis inhibitor (TAFI)), irrespective of the presence of lectins. MASP-1 initiates fibrin clot formation in a thrombin-dependent reaction by converting prothrombin to thrombin which then cleaves fibrinogen. MASP-1 also catalyses fibrin cross-linking by activating FXIII independently from thrombin, albeit less efficiently (having a greater effect on Val34 variant compared with Leu34, which is the opposite to thrombin). In addition to its prothrombotic effects, TAFI activation by MASP-1 offers protection from clot lysis as shown by prolongation of clot lysis time in the presence of MASP-1. TAFI is a potent antifibrinolytic proenzyme which is physiologically activated by thrombin, plasmin, or the thrombin/thrombomodulin complex [64]. Intriguingly, the activated enzyme, TAFIa, exerts its antifibrinolytic actions in a manner that closely resembles lysine analogues (removes lysine residues on fibrin which eliminates the binding sites for plasminogen) [65]. Jenny et al [66] demonstrated that MASP-1 gives rise to an alternative active form of thrombin by cleaving prothrombin at the cleavage site Arg393; and despite that MASP-1 is the exclusive activator of MASP-2 [67], MASP-2 has no effect on the capability of MASP-1 to promote clotting as shown by lack of response to MASP-2 inhibition. By contrast, inhibition of the proteolytic activity of MASP-1 prevents activation of MASP-2 [68]. Noteworthy, MASP-1 lacks any clotting activity in the absence of prothrombin.
Finally, as MASP-1 procoagulant profile differs from MASP-2, this might have therapeutic implications. For instance, it might be more practical to use lectin-MASP-2 conjugates for local purposes (like open wounds) where systemic thrombosis is not needed while utilizing MASP-1 for patients with traumatic/surgical bleeds. If this hypothesis is validated, these MASPs could be a promising therapeutic strategy for the prophylaxis/management of bleeding. In addition, they can also be contemplated for the management of bleeding in patients with hemophilia A and B where the basic biochemical abnormality is the inability to activate FX that generate thrombin and fibrin in order to stabilize the platelet clot [69]. In summary, the complement and coagulation systems must be viewed as inextricably intertwined [70]. MASP-1 and MASP-2 link innate immunity to secondary hemostasis, and by virtue of their hemostatic potential, are promising novel agents for the treatment and/or prevention of acute bleeding.
Acknowledgments
None to declare.
Financial Disclosure
Author has no financial disclosure to report.
Conflict of Interest
The author declares no conflict of interest.
Informed Consent
Not applicable.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
doi - Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
doi - Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed - Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099.
doi pubmed - Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040.
doi pubmed - Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17):e38.
doi pubmed - Bumiller-Bini V, de Freitas Oliveira-Tore C, Carvalho TM, Kretzschmar GC, Goncalves LB, Alencar NM, Gasparetto Filho MA, et al. MASPs at the crossroad between the complement and the coagulation cascades - the case for COVID-19. Genet Mol Biol. 2021;44(Suppl 1):1 e20200199.
doi pubmed - Jenny L, Dobo J, Gal P, Pal G, Lam WA, Schroeder V. MASP-1 of the complement system enhances clot formation in a microvascular whole blood flow model. PLoS One. 2018;13(1):e0191292.
doi pubmed - Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507-1511.
doi pubmed - Leal-Noval SR, Fernandez Pacheco J, Casado Mendez M, Cuenca-Apolo D, Munoz-Gomez M. Current perspective on fibrinogen concentrate in critical bleeding. Expert Rev Clin Pharmacol. 2020;13(7):761-778.
doi pubmed - Johnston SS, Jamous N, Mistry S, Jain S, Gangoli G, Danker W, Ammann E, et al. Association of in-hospital surgical bleeding events with prolonged hospital length of stay, days spent in critical care, complications, and mortality: a retrospective cohort study among patients undergoing neoplasm-directed surgeries in English hospitals. Clinicoecon Outcomes Res. 2021;13:19-29.
doi pubmed - Cata JP, Vijaya Gottumukkala V. Blood loss and massive transfusion in patients undergoing major oncological surgery: what do we know? ISRN Anesthesiol. 2012;2012:1-11.
doi - Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med. 2018;7(2):265-273.
doi pubmed - Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist. 2004;9(5):561-570.
doi pubmed - Sniecinski RM, Levy JH. Bleeding and management of coagulopathy. J Thorac Cardiovasc Surg. 2011;142(3):662-667.
doi pubmed - Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298-304.
doi pubmed - Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14(2):R52.
doi pubmed - Moore EE, Knudson MM, Jurkovich GJ, Fildes JJ, Meredith JW. Emergency traumatologist or trauma and acute care surgeon: decision time. J Am Coll Surg. 2009;209(3):394-395.
doi pubmed - MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39-44.
doi pubmed - Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127-1130.
doi pubmed - Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10(1):23-27.
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748-754.
doi pubmed - Schulman S. Pharmacologic tools to reduce bleeding in surgery. Hematology Am Soc Hematol Educ Program. 2012;2012:517-521.
doi pubmed - Cimmino G, Cirillo P. Tissue factor: newer concepts in thrombosis and its role beyond thrombosis and hemostasis. Cardiovasc Diagn Ther. 2018;8(5):581-593.
doi pubmed - Borensztajn K, Spek CA. Blood coagulation factor Xa as an emerging drug target. Expert Opin Ther Targets. 2011;15(3):341-349.
doi pubmed - Chen A, Stecker E, B AW. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559.
doi pubmed - Julia S, James U. Direct Oral Anticoagulants: A Quick Guide. Eur Cardiol. 2017;12(1):40-45.
doi - Wood JP, Silveira JR, Maille NM, Haynes LM, Tracy PB. Prothrombin activation on the activated platelet surface optimizes expression of procoagulant activity. Blood. 2011;117(5):1710-1718.
doi pubmed - Fredenburgh JC, Weitz JI. Overview of hemostasis and thrombosis. Hematology. 2018. p. 1831-1842.
doi - Haynes LM, Bouchard BA, Tracy PB, Mann KG. Prothrombin activation by platelet-associated prothrombinase proceeds through the prethrombin-2 pathway via a concerted mechanism. J Biol Chem. 2012;287(46):38647-38655.
doi pubmed - Bouchard BA, Silveira JR, Tracy PB. Interactions between platelets and the coagulation system. Michelson AD (Ed.). Platelets. Academic Press; San Diego, CA. 2013. p. 425-451.
doi - Fager AM, Wood JP, Bouchard BA, Feng P, Tracy PB. Properties of procoagulant platelets: defining and characterizing the subpopulation binding a functional prothrombinase. Arterioscler Thromb Vasc Biol. 2010;30(12):2400-2407.
doi pubmed - Krishnaswamy S. The transition of prothrombin to thrombin. J Thromb Haemost. 2013;11(Suppl 1):265-276.
doi pubmed - Ekdahl KN, Teramura Y, Hamad OA, Asif S, Duehrkop C, Fromell K, Gustafson E, et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274(1):245-269.
doi pubmed - Conway EM. Reincarnation of ancient links between coagulation and complement. J Thromb Haemost. 2015;13(Suppl 1):S121-132.
doi pubmed - Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement—their role in inflammation. Semin Immunopathol. 2012;34(1):151-165.
doi pubmed - Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185(9):5628-5636.
doi pubmed - Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184-192.
doi pubmed - Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-797.
doi pubmed - Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005;3(8):1861-1872.
doi pubmed - Matsushita M, Kuraya M, Hamasaki N, Tsujimura M, Shiraki H, Fujita T. Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J Immunol. 2002;168(7):3502-3506.
doi pubmed - Dobo J, Szakacs D, Oroszlan G, Kortvely E, Kiss B, Boros E, Szasz R, et al. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep. 2016;6:31877.
doi pubmed - Gulla KC, Gupta K, Krarup A, Gal P, Schwaeble WJ, Sim RB, O'Connor CD, et al. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology. 2010;129(4):482-495.
doi pubmed - Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13.
doi pubmed - Yongqing T, Drentin N, Duncan RC, Wijeyewickrema LC, Pike RN. Mannose-binding lectin serine proteases and associated proteins of the lectin pathway of complement: two genes, five proteins and many functions? Biochim Biophys Acta. 2012;1824(1):253-262.
doi pubmed - Demopulos GA, Dudler T, Nilsson B. Omeros Corporation Assignee. Compositions and methods of inhibiting MASP-2 for the treatment of various thrombotic diseases and disorders. Patent WO 2019/246367 A1. 2019.
- van Emmerik LC, Kuijper EJ, Fijen CA, Dankert J, Thiel S. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin Exp Immunol. 1994;97(3):411-416.
doi pubmed - Elhadad S, Chapin J, Copertino D, Van Besien K, Ahamed J, Laurence J. MASP2 levels are elevated in thrombotic microangiopathies: association with microvascular endothelial cell injury and suppression by anti-MASP2 antibody narsoplimab. Clin Exp Immunol. 2021;203(1):96-104.
doi pubmed - Ali YM, Ferrari M, Lynch NJ, Yaseen S, Dudler T, Gragerov S, Demopulos G, et al. Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front Immunol. 2021;12:714511.
doi pubmed - Ardissino G, Capone V, Tedeschi S, Porcaro L, Cugno M. Complement System as a New Target for Hematopoietic Stem Cell Transplantation-Related Thrombotic Microangiopathy. Pharmaceuticals (Basel). 2022;15(7):845.
doi pubmed - Gavriilaki E, Ho VT, Schwaeble W, Dudler T, Daha M, Fujita T, Jodele S. Role of the lectin pathway of complement in hematopoietic stem cell transplantation-associated endothelial injury and thrombotic microangiopathy. Exp Hematol Oncol. 2021;10(1):57.
doi pubmed - Safety and efficacy study of OMS721 in patients with thrombotic microangiopathies. https://clinicaltrials.gov/ct2/show/NCT02222545. Accessed Nov 16, 2021.
- Khaled SK, Boelens JJ, Cairo MS, Champlin R, Duarte RF, Giralt SA, et al. Narsoplimab (OMS721), a MASP-2 inhibitor, for the treatment of adult hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA). Transplant Cell Ther. 2021;27(3 Supplement):S24-S26.
doi - Rambaldi A, Gritti G, Mico MC, Frigeni M, Borleri G, Salvi A, Landi F, et al. Endothelial injury and thrombotic microangiopathy in COVID-19: Treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225(6):152001.
doi pubmed - Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2(7):e623.
doi pubmed - Ashraf Abdullah Saad. Targeting cancer-associated glycans as a therapeutic strategy Leukemia. All Life. 2022;15(1):378-433.
doi - Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284.
doi pubmed - Preston RJ, Rawley O, Gleeson EM, O'Donnell JS. Elucidating the role of carbohydrate determinants in regulating hemostasis: insights and opportunities. Blood. 2013;121(19):3801-3810.
doi pubmed - Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J Biol Chem. 2001;276(44):40880-40887.
doi pubmed - Ambrus G, Gal P, Kojima M, Szilagyi K, Balczer J, Antal J, Graf L, et al. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J Immunol. 2003;170(3):1374-1382.
doi pubmed - Dobo J, Harmat V, Beinrohr L, Sebestyen E, Zavodszky P, Gal P. MASP-1, a promiscuous complement protease: structure of its catalytic region reveals the basis of its broad specificity. J Immunol. 2009;183(2):1207-1214.
doi pubmed - Presanis JS, Hajela K, Ambrus G, Gal P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Mol Immunol. 2004;40(13):921-929.
doi pubmed - Hess K, Ajjan R, Phoenix F, Dobo J, Gal P, Schroeder V. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS One. 2012;7(4):e35690.
doi pubmed - Sillen M, Declerck PJ. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int J Mol Sci. 2021;22(7): 3670.
doi pubmed - Bajzar L, Jain N, Wang P, Walker JB. Thrombin activatable fibrinolysis inhibitor: not just an inhibitor of fibrinolysis. Crit Care Med. 2004;32(5 Suppl):S320-S324.
doi pubmed - Jenny L, Dobó J, Gál P, Schroeder V. MASP-1 of the complement system promotes clotting via prothrombin activation. Mol Immunol. 2015;65(2):398-405.
doi pubmed - Heja D, Kocsis A, Dobo J, Szilagyi K, Szasz R, Zavodszky P, Pal G, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci U S A. 2012;109(26):10498-10503.
doi pubmed - Parej K, Dobo J, Zavodszky P, Gal P. The control of the complement lectin pathway activation revisited: both C1-inhibitor and antithrombin are likely physiological inhibitors, while α2-macroglobulin is not. Mol Immunol. 2013;54(3-4):415-422.
doi pubmed - Kumar R, Carcao M. Inherited abnormalities of coagulation: hemophilia, von Willebrand disease, and beyond. Pediatr Clin North Am. 2013;60(6):1419-1441.
doi pubmed - Pryzdial ELG, Leatherdale A, Conway EM. Coagulation and complement: Key innate defense participants in a seamless web. Front Immunol. 2022;13:918775.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


