| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 1, February 2023, pages 37-41
Soft-Tissue Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma in a Child Unmasked by COVID-19
Diego Alberto Lozano-Jaramilloa, b, c, Esperanza Millan-Arreolab, Oscar Omar Esquer-Cotab, Jesus Manuel Lozano-Garciab, Miguel Alfonso Valenzuela-Espinozab
aCentro de Investigacion Valle Bibb Fundacion, Tijuana, Baja California, Mexico
bCentro Oncologico Pediatrico de Baja California, Tijuana, Baja California, Mexico
cCorresponding Author: Diego Alberto Lozano-Jaramillo, Centro de Investigacion Valle Bibb Fundacion, Tijuana, Baja California, Mexico
Manuscript submitted December 7, 2022, accepted January 26, 2023, published online February 25, 2023
Short title: ALK-Positive ALCL Unmasked by COVID-19
doi: https://doi.org/10.14740/jh1081
| Abstract | ▴Top |
Anaplastic large cell lymphoma (ALCL) is children’s most common mature T-cell neoplasm. The majority is positive for anaplastic lymphoma kinase (ALK). Initial presentation as a soft-tissue pelvic mass without nodal involvement is rare and can be easily misdiagnosed. We report a case of a 12-year-old male presenting with pain and movement restriction in the right extremity. Computed tomography (CT) scan revealed a solitary pelvic mass. Initial biopsy examination concluded rhabdomyosarcoma. After developing pediatric multisystemic inflammatory syndrome due to coronavirus disease 2019 (COVID-19), central and peripheral lymph node enlargement appeared. New cervical adenopathy and pelvic mass biopsies were performed. Immunohistochemistry concluded an ALK-positive ALCL with a small-cell pattern. The patient was treated with brentuximab-based chemotherapy and eventually improved. Differential diagnosis of pelvic masses in children and adolescents must include ALCL. An inflammatory trigger may promote the appearance of a typical nodal disease, previously absent. Attention is warranted during histopathological examination to avoid diagnostic errors.
Keywords: Anaplastic large cell lymphoma; Anaplastic lymphoma kinase; COVID-19; Rhabdomyosarcoma
| Introduction | ▴Top |
Lymphoma represents the third most common malignancy in childhood, with non-Hodgkin lymphoma (NHL) accounting for 7% of all cancers in patients younger than 20 years old [1]. Most of NHL subtypes in childhood are high-grade lymphomas [2]. Children’s most common mature T-cell neoplasm is anaplastic large cell lymphoma (ALCL), representing 15% of all NHL [3]. ALCL is a peripheral T-cell lymphoma characterized by its eccentric, horseshoe-shaped nuclei and strongly expressed CD30. Chromosomal translocation t (2:5) is recurrently present. This translocation involves a receptor tyrosine kinase named anaplastic lymphoma kinase (ALK) and nucleophosmin (NPM) [4]. To detect the presence of ALK, anti-ALK antibodies are used in immunohistochemical analysis as a surrogate for t (2:5) [5]. Current World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues divides ALCL into two large groups based on the presence or absence of ALK expression [6]. In contrast to ALK-positive ALCL, ALK-negative ALCL occurs predominantly in adults and has an overall poorer outcome. ALK-positive ALCL represents more than 90% of cases in children [7].
We present the case of a 12-year-old-male with a soft-tissue pelvic mass with the initial diagnosis of rhabdomyosarcoma (RMS). After a severe coronavirus disease 2019 (COVID-19) infection, peripheral adenopathies appeared. New biopsies were performed, and immunohistochemical studies revealed an ALK-positive ALCL with a small cell pattern.
| Case Report | ▴Top |
Investigations
A previously healthy 12-year-old male presented with no relevant medical history, with paresthesia, pain, and arc-movement limitation in the lower right extremity. He was initially treated with non-steroid anti-inflammatory drugs with mild improvement.
Two months later, the pain became persistent. The patient’s mother noted a mass on the right groin area, evident at physical examination, with no other relevant findings. Laboratory tests and abdominopelvic computed tomography (CT) scan were performed. Laboratory results yield hemoglobin levels at 4.4 g/dL, mean corpuscular volume at 76.90 fL, ferritin levels at 4.2 µg/L and a transferrin saturation at 8.23%, compatible with severe iron-deficiency anemia. Liver function tests and creatinine were normal, including lactate dehydrogenase at 225 IU/L. CT scan showed a tumor mass on the right iliopsoas with no evident adenopathies (Fig. 1). Blood transfusion was given immediately, and an ultrasound-guided Tru-Cut pelvic mass biopsy was performed. The pathology department reported a small round blue cell tumor. RMS was the initial presumptive diagnosis based on morphology alone with pending immunohistochemistry.
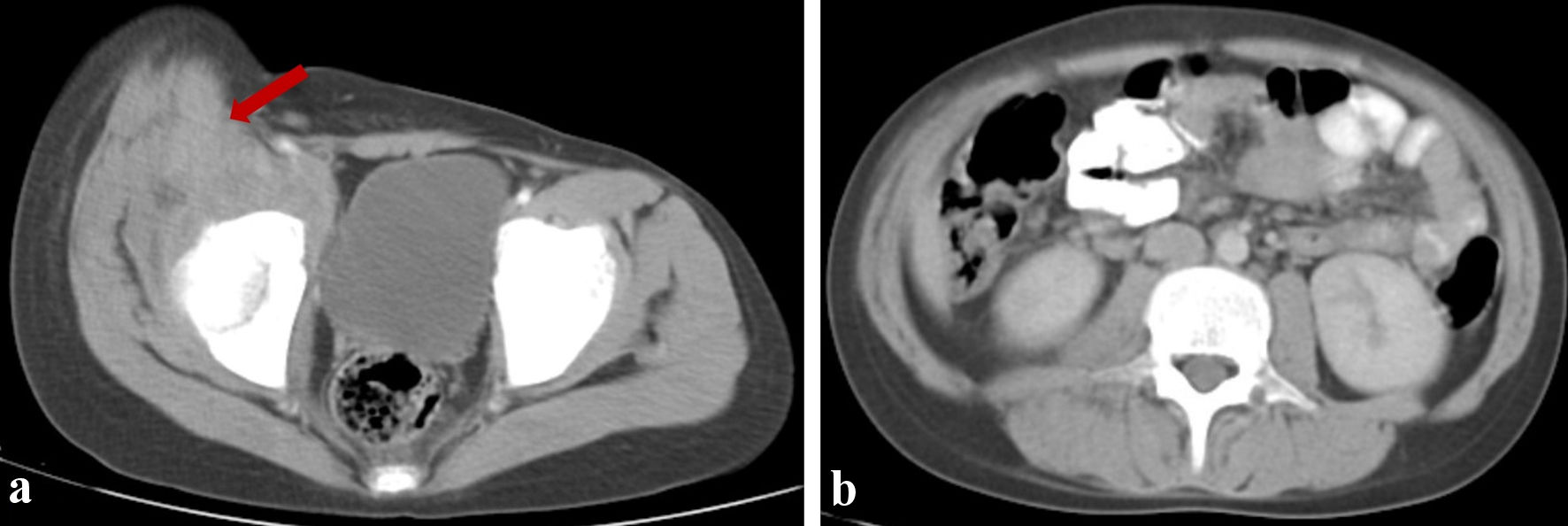 Click for large image | Figure 1. Initial CT scan images. (a) A pelvic mass located in the iliopsoas region (arrow). (b) Abdominal CT scan showing no retroperitoneal lymph node enlargement. CT: computed tomography. |
During hospitalization, the patient developed upper gastrointestinal bleeding. Endoscopy revealed a submucosal ulcerated and pedunculated lesion in the gastric body and greater curvature, suggestive of a gastrointestinal stromal tumor. New biopsies were performed.
Eight days after arrival, mild to moderate respiratory symptoms were noted with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen test. Four days later, the patient suffered from dyspnea followed by the appearance of multiple enlarged lymph nodes in the cervical, supraclavicular and groin regions, previously absent. A complete CT scan revealed cervical, mesenteric, and retro-peritoneal adenopathies, bilateral pleural effusion, and ground-glass opacities in lung parenchyma (Fig. 2). A chest tube was placed and node biopsies were taken.
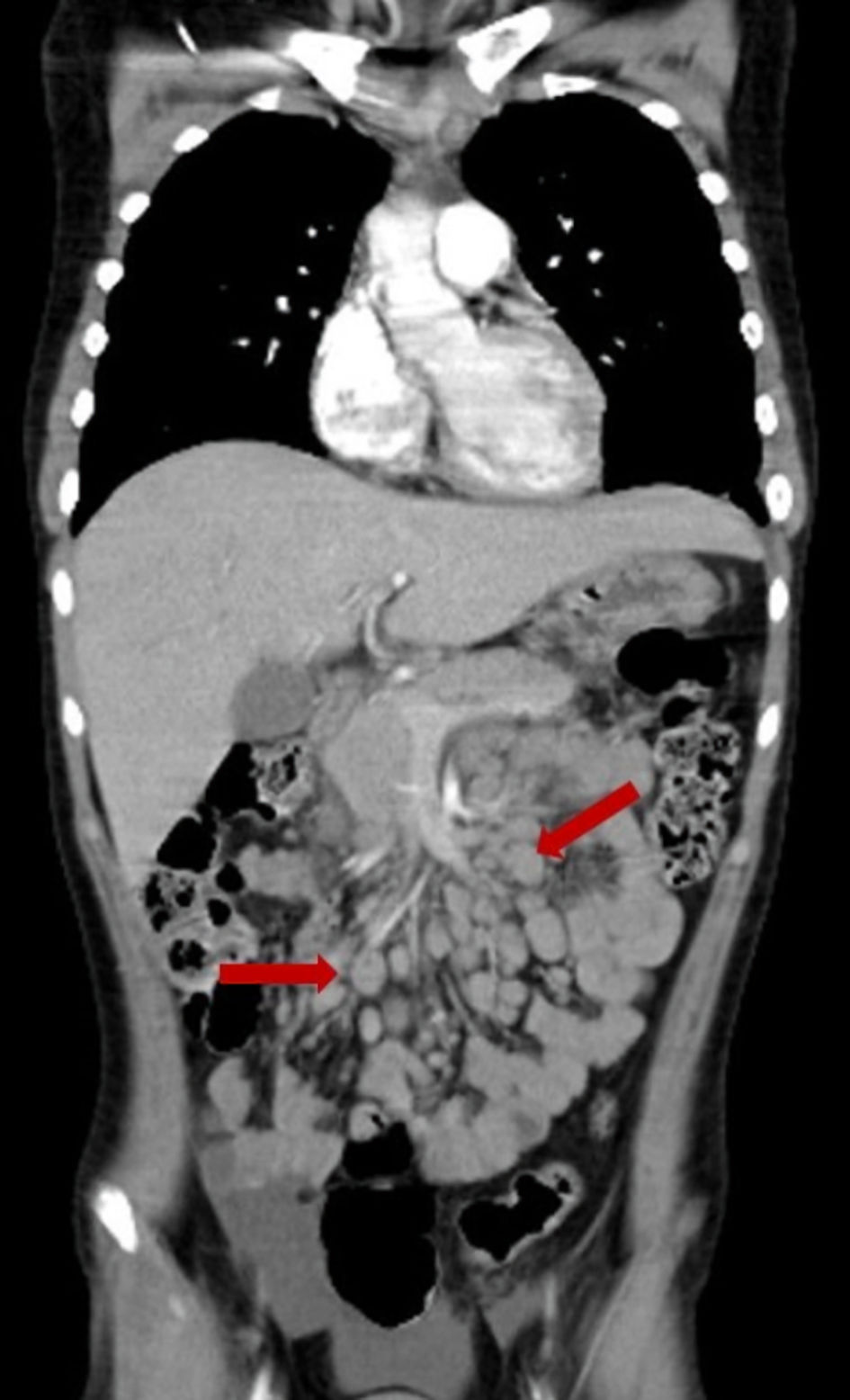 Click for large image | Figure 2. Coronal CT scan of the abdomen showing multiple retroperitoneal adenopathies (arrows). CT: computed tomography. |
Diagnosis
Preliminary analysis of the pelvic mass, gastric, and the recent nodal biopsies reported a poorly differentiated hematopoietic neoplasm. Immunohistochemistry showed CD45 positive markers in all tumor cells, terminal deoxynucleotidyl transferase (Tdt) negative, myeloperoxidase (MPO) negative, paired box 5 (PAX-5) negative, CD3 negative, CD30 perimembranous positive, and ALK-positive in 100% of tumor cells. ALK-positive ALCL with a small-cell pattern was concluded (Fig. 3).
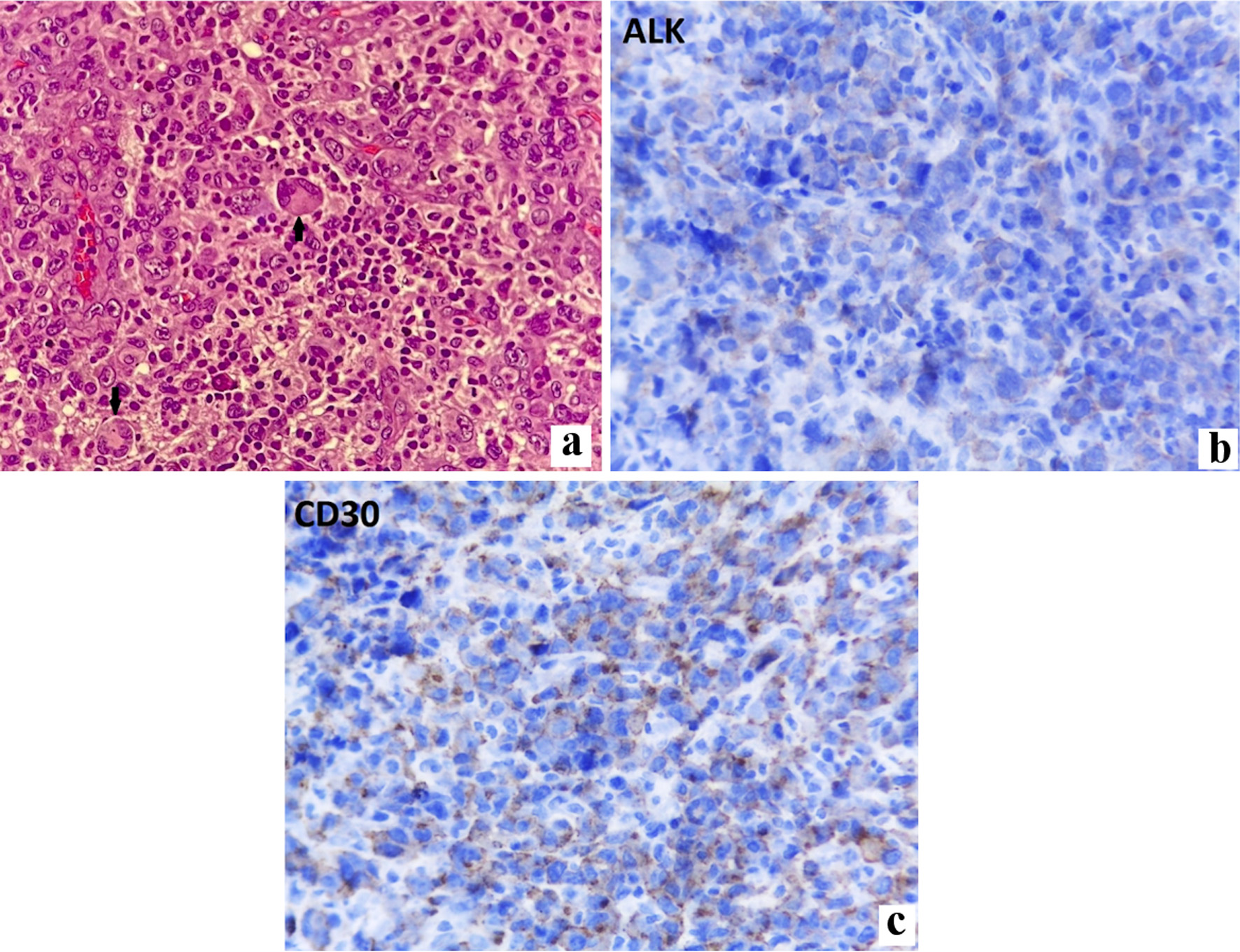 Click for large image | Figure 3. (a) Hematoxylin and eosin stain of soft-tissue mass showing a small-lymphocyte pattern and hallmark cells (arrows). Immunohistochemistry stained positively for ALK (b) and CD30 (c) markers. ALK: anaplastic lymphoma kinase |
Treatment
Broad-spectrum antibiotics were initiated due to a positive pleural fluid culture for gram-positive cocci. Pediatric multisystem inflammatory syndrome criteria were fulfilled, and treatment with gamma globulin was initiated. Brentuximab-based chemotherapy protocol was prescribed according to the ANHL12P1 trial by Children’s Oncology Group (COG).
Follow-up and outcomes
The patient eventually developed multifactorial acute renal failure due to methotrexate intoxication, tumor lysis syndrome, and severe sepsis. The patient was started on hemodialysis, along with folinic acid rescue therapy. The patient showed progressive clinical and laboratory improvement after a few days of treatment. He was eventually discharged with no significant residuals. As of today, he is still being treated with the same brentuximab-based regimen with significant clinical and laboratory improvement.
| Discussion | ▴Top |
The diagnosis of soft-tissue tumors can represent a challenge in pediatric and adolescent patients. RMS is the most common soft-tissue tumor in childhood, representing 4.5% of all childhood cancer. It typically presents as an asymptomatic mass [8]. The most common differential diagnoses for a pelvic mass in children and adolescents are RMS, Ewing’s sarcoma, and osteosarcoma [9]. Case reports were found where patients with soft-tissue masses were initially misdiagnosed before concluding ALCL, as in our patient [10, 11]. Attention is warranted when microscopically evaluating poorly differentiated soft-tissue tumors.
Clinically, ALK-positive ALCL commonly presents at an advanced stage (III - IV) with B symptoms. Association of extranodal sites is relatively common, with up to 50% of bone marrow involvement detected by reverse transcription-polymerase chain reaction (RT-PCR) [6, 12]. In a report by d’Amore et al, up to 46% of patients had extranodal involvement, defined as at least one of the following sites: visceral involvement, skin, bone marrow, bone, soft-tissue, central nervous system; in contrast, 90% had a typical nodal presentation of lymphoma [13]. The Children’s Cancer Study, from the United Kingdom group, analyzed 72 patients from 1990 to 1996, with a median age of 11.8 years who were treated for ALCL. Of these, 40% had extranodal disease. Only six patients had soft-tissue involvement, and nine had bone disease [14]. The exact percentage of patients with pelvic mass as the initial form of presentation of an ALK-positive ALCL is unknown, but it seems rare.
A case report and review by Kounami et al found that up to 2012, only eight cases of ALCL arising from the musculoskeletal system were found. All were children or adolescents except one, most being ALK-positive. They concluded that it is difficult to assess the prognosis of these patients given the small number of reported cases [15].
The ALCL99 trial determined the prognostic factors involved in ALCL in childhood. Four hundred twenty patients were analyzed. Of these, only 37 had soft-tissue mass at diagnosis. The 10-year progression-free survival was 81% vs. 68% for those with and without soft-tissue mass, respectively, although the P value was non-significant (0.22). The only clinical/pathological characteristic associated with the risk of failure (hazard ratio (HR): 2.49) was a small cell/lymphohistiocytic pattern [16]. Our patient had a small cell/lymphohistiocytic pattern. No studies suggest a poorer prognosis when a soft-tissue mass is involved.
It was previously reported that ALK-positive cutaneous ALCL can manifest after an insect bite [17]. In our case at diagnosis the only tumor mass identified by the CT scan was the pelvic one with no adenopathies. After COVID-19, the node enlargements appeared, as in a typical nodal-involved lymphoma. We do not suggest COVID-19 as the initial pathogenic driver for the development of ALK-positive ALCL, rather as a possible trigger that promoted the node enlargement and made the clinical picture clearer. The exact mechanisms of how COVID-19 might have promoted this is unknown, but it can be assumed that cytokine-driven inflammatory response was critical. More studies are needed to understand the exact mechanisms.
There are multiple treatment strategies available for ALK-positive ALCL. It is regarded as a chemo-sensitive lymphoma with overall survival (OS) ranging from 70% to 90% and event-free survival (EFS) between 65% and 75%. Extranodal disease, advanced stage, or soft-tissue involvement do not influence the initial treatment strategies [18, 19].
Learning points
ALK-positive ALCL must be considered as a differential diagnosis in solitary pelvic masses in children and adolescents. Immunohistochemistry requires expert evaluation in order to reach a precise diagnosis. As demonstrated in this case report, a systemic response after an inflammatory trigger could unmask the clinical picture as a typical nodal-involved lymphoma.
Acknowledgments
We want to thank all the hard-working people at Centro Oncologico Pediatrico and Fundacion Castro-Limon.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Written informed consent has been obtained.
Author Contributions
Lozano-Jaramillo, Millan-Arreola and Esquer-Cota conceived and conceptualized the idea. Lozano-Jaramillo, Esquer-Cota and Lozano-Garcia wrote the initial draft. Valenzuela-Espinoza and Millan-Arreola review and edited the subsequent drafts. All authors helped with the visualization and have read and agreed to publish the final version of the manuscript.
Data Availability
The data used in this study were accurately cited within the manuscript. Further inquiries can be directed to the corresponding author.
Abbreviations
ALCL: anaplastic large cell lymphoma; ALK: anaplastic lymphoma kinase; CT: computed tomography; COVID-19: coronavirus disease of 2019; COG: Children’s Oncology Group; EFS: event-free survival; OS: overall survival; NHL: non-Hodgkin lymphoma; RMS: rhabdomyosarcoma; RT-PCR: reverse transcription-polymerase chain reaction; WHO: World Health Organization
| References | ▴Top |
- Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3(3):167-173.
doi pubmed - Sandlund JT, Martin MG. Non-Hodgkin lymphoma across the pediatric and adolescent and young adult age spectrum. Hematology Am Soc Hematol Educ Program. 2016;2016(1):589-597.
doi pubmed - Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013;30(6):509-519.
doi pubmed - Kinney MC, Higgins RA, Medina EA. Anaplastic large cell lymphoma: twenty-five years of discovery. Arch Pathol Lab Med. 2011;135(1):19-43.
doi pubmed - Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997;89(4):1394-1404.
doi pubmed - Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748.
doi pubmed - Pina-Oviedo S, Ortiz-Hidalgo C, Carballo-Zarate AA, Zarate-Osorno A. ALK-negative anaplastic large cell lymphoma: current concepts and molecular pathogenesis of a heterogeneous group of large T-cell lymphomas. Cancers (Basel). 2021;13(18):4667.
doi pubmed - Dasgupta R, Fuchs J, Rodeberg D. Rhabdomyosarcoma. Semin Pediatr Surg. 2016;25(5):276-283.
doi pubmed - Williams RF, Fernandez-Pineda I, Gosain A. Pediatric sarcomas. Surg Clin North Am. 2016;96(5):1107-1125.
doi pubmed - Gupta D, Vishwajeet V, Ramzan M, Elhence PA. ALK-positive anaplastic large cell lymphoma of skeletal muscle masquerading as soft tissue sarcoma. BMJ Case Rep. 2022;15(2):e245685.
doi pubmed - Sriphongphankul H, Tanpowpong P, Ruangwattanapaisarn N, Thirapattaraphan C, Treepongkaruna S. Anaplastic large cell lymphoma of the duodenum in a teenage girl: misdiagnosed as an intramural duodenal hematoma. Pediatr Gastroenterol Hepatol Nutr. 2019;22(6):571-575.
doi pubmed - Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
doi pubmed - d'Amore ES, Menin A, Bonoldi E, Bevilacqua P, Cazzavillan S, Donofrio V, Gambini C, et al. Anaplastic large cell lymphomas: a study of 75 pediatric patients. Pediatr Dev Pathol. 2007;10(3):181-191.
doi pubmed - Williams DM, Hobson R, Imeson J, Gerrard M, McCarthy K, Pinkerton CR, United Kingdom Children's Cancer Study G. Anaplastic large cell lymphoma in childhood: analysis of 72 patients treated on the United Kingdom Children's Cancer Study Group chemotherapy regimens. Br J Haematol. 2002;117(4):812-820.
doi pubmed - Kounami S, Shibuta K, Yoshiyama M, Mitani Y, Watanabe T, Takifuji K, Yoshikawa N. Primary anaplastic large cell lymphoma of the psoas muscle: a case report and literature review. Acta Haematol. 2012;127(3):186-188.
doi pubmed - Mussolin L, Le Deley MC, Carraro E, Damm-Welk C, Attarbaschi A, Williams D, Burke A, et al. Prognostic factors in childhood anaplastic large cell lymphoma: long term results of the international ALCL99 trial. Cancers (Basel). 2020;12(10):2747.
doi pubmed - Lamant L, Pileri S, Sabattini E, Brugieres L, Jaffe ES, Delsol G. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: evidence for an association in five cases. Haematologica. 2010;95(3):449-455.
doi pubmed - Prokoph N, Larose H, Lim MS, Burke GAA, Turner SD. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): current standard and beyond. Cancers (Basel). 2018;10(4):99.
doi pubmed - Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, Wu R, et al. Brentuximab vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: results of COG trial ANHL12P1. Blood. 2021;137(26):3595-3603.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


