| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 2, Number 2, October 2013, pages 55-63
Experience Always Wins the Day: Measure of Warfarin Anticoagulation Efficiency in the Internal Medicine Clinic
Thi Hoang Lan Nguyena, c, Nathalye Belzilea, c, Madeleine Durandb, Josee Leroux-Stewarta, Stephanie Thebeaua, Oceane Landon-Cardinala, Robert Wistaffb, Paul-Van Nguyenb, Maxime Lamarre-Clicheb, Christoph Kolanb, Mikhael Laskineb, d
aPGY3 Internal Medicine Programme, Hotel-Dieu Hospital, Centre Hospitalier de l’Universite de Montreal, Universite de Montreal, Canada
bInternal Medicine, Hotel-Dieu Hospital, Centre Hospitalier de l’Universite de Montreal, Universite de Montreal, Canada
cThese authors contributed equally to this work
dCorresponding author: Mikhael Laskine, Hotel-Dieu Hospital, CHUM, 3840 St-Urbain str, Montreal, Qc, Canada
Manuscript accepted for publication August 28, 2013
Short title: Experience Always Wins Day
doi: https://doi.org/10.4021/jh100e
| Abstract | ▴Top |
Background: In the real-life setting of a specialized anticoagulation clinic we think that the patient’s INRs fall within the therapeutical range most of the time. Our study aimed to assess the time spent by patients treated by Warfarin with INR between 2.0 and 3.0, as well the time spent between 1.5 and 3.5. We chose this new range (1.5 - 3.5) because we think that it is safe and completely acceptable in real-life situation, and requires only minor adjustments to return to treatment goal of 2.0 to 3.0.
Methods: A single-center, retrospective observational study was conducted at Hotel-Dieu Hospital, between June 2010 and December 2011. Inclusion criteria were (1) to be treated for venous thromboembolic event with Warfarin, and (2) to have at least 3 INR measurements during the study period.
Results: The median duration of follow-up was 281 days with a total number of INR values of 2,553. Median proportion of time at target INR (2.0 - 3.0) was 68.9%. This proportion increased to 98.7% between 1.5 and 3.5. There was a single recurrent thrombosis event, 6 minor bleeding episodes and 10 major bleeding episodes. The majority of major bleeding episodes were caused by gastrointestinal bleeding.
Conclusions: We demonstrated that patients followed at a specialized anticoagulation clinic spend on average 68.9% of their time within the therapeutical range of INR and 98.7% of their time within very safe and effective INR values. Indeed, Warfarin is still a valuable treatment for thromboembolic event and a very competitive drug.
Keywords: Warfarin; Anticoagulation therapy; Thromboembolic event; INR; Therapeutic range
| Introduction | ▴Top |
Warfarin has been used for the treatment of thromboembolic events (TE) for decades. Since the introduction of new oral anticoagulants, this treatment seems a less pragmatic choice. Indeed, the narrowed therapeutic index and the numerous intrinsic and extrinsic interactions make Warfarin less appealing to clinicians [1-3]. However, Warfarin remains the most studied anticoagulant available, as well as the only oral agent with an effective antidote.
Warfarin necessitates serial follow-up, which, although most often seen as an encumbrance, can be an advantage, especially in an ageing population with many comorbidities. For one thing, serial International normalized ratios (INR) are a measure of observance to treatment, as well as informative to clinicians about the thrombosis and bleeding risks of patients. In addition, advantages of Warfarin over newer anticoagulants include availability of antidote in case of bleeding, and safe, effective, and inexpensive treatment.
In most large randomized controlled studies, the proportion of INR values falling within the chosen therapeutic range of 2.0 - 3.0 is relatively low. In the EINSTEIN-DVT and PE studies, the proportion of INR values falling within this target range were 57.7% and 62.7% respectively, suggesting newer anticoagulants were superior because they seems to provide anticoagulation within therapeutic target at all times [4, 5]. We think that this should not be considered to discourage the use of warfarin.
We argue that time spent within the therapeutic range of INR, rather than the percent of values falling within that range, is important to measure. In the real-life setting of a specialized anticoagulation clinic, the patient’s INRs fall within the therapeutical range most of the time. We also believe that, although the well-established INR therapeutical range is 2.0 to 3.0, the range between INRs of 1.5 and 3.5 is safe, and requires only minor adjustments to return to treatment goals of 2.0 to 3.0.
We aimed to assess the time spent by patients followed at our anticoagulation clinic with INR between 2.0 and 3.0, as well the time spent very close to those therapeutic targets (1.5 to 2.0 and 3.0 to 3.5).
| Materials and Methods | ▴Top |
Study design
We conducted a single-center, retrospective observational cohort study at Hopital Hotel-Dieu (HDM), Centre Hospitalier de l’Universite de Montreal (CHUM) between June 2010 and December 2011. All adult (≥ 18 year-old) patients followed at the anticoagulation clinic of the department of Internal Medicine during the study period were included. Inclusion criteria were as follows (a) to be treated for venous TE event (either deep vein thrombosis (DVT) or pulmonary embolism (PE)) with Warfarin, and (b) to have at least 3 INR measurements during the study period.
Patients were excluded from the study if they were anticoagulated for reasons that differed from inclusion criteria. They were also excluded if they were not on Warfarin at the time of the study, were anticoagulated with anti-Xa, direct thrombin inhibitor drugs or low molecular weight heparin (LMWH). Patients with antiphospholipid syndrome with target INR of 2.5 to 3.5 were also excluded.
For each patient, we collected data for a maximum of one year from either the beginning of the study period (prevalent TE cases) or the occurrence of the TE event (incident TE cases). Patients’ demographic and clinical characteristics, laboratory tests results, use of antiplatelet drugs, concomitant low molecular weight heparin administration, thromboembolic and hemorrhagic events were collected from the hospital electronic chart system and the anticoagulation clinic charts.
The co-primary outcomes were the proportion of time spent with INR between 2.0 and 3.0 and between 1.5 and 3.5, as measured with the linear interpolation method described by Rosendaal et al [6]. This method measures the proportion of time spent between two INR values, rather than focusing on the ratio of INR values lying between two values. This method gives a better picture of the extent of therapeutical anticoagulation with Warfarin, while capturing the impact of extreme values of INR. Figure 1 illustrates the differences between the linear interpolation and proportional methods.
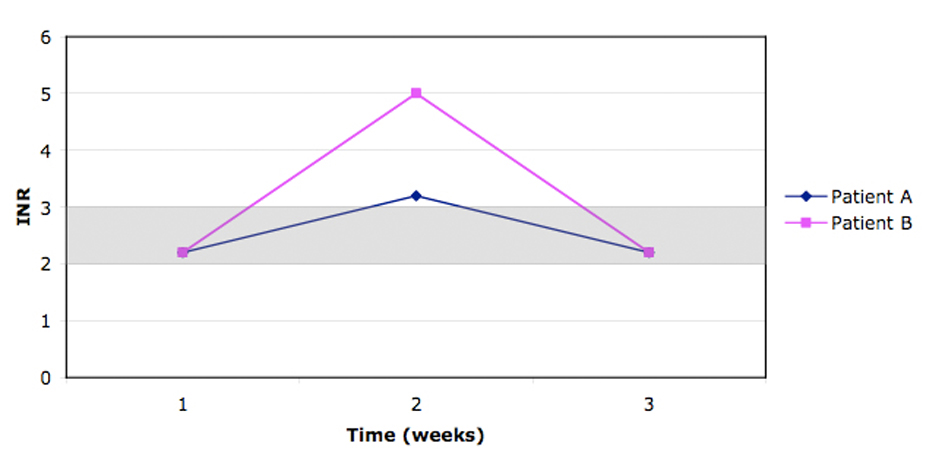 Click for large image | Figure 1. During the 2 weeks follow-up, when using linear interpolation, patient A remains in the therapeutic index (2.0 - 3.0) during 80% of the time (11.2 days) vs patient B who only remains 21% of the time (3 days). Comparatively when using the proportional method, both patients stay in the therapeutic index 67% of the time (2/3 values within 2.0 - 3.0). |
Secondary outcomes were the proportion of INR values between 2.0 and 3.0 in different groups, the occurrence of recurrent TE events, any bleeding and major bleeding events.
Recurrent TE events were defined as recurrence of symptomatic DVT of the lower or upper extremities or PE, while on treatment with Warfarin. Recurrent events had to be confirmed by Doppler examination, pulmonary ventilation/perfusion scan or pulmonary computed tomography with infusion of contrast media. Any bleeding was defined as inpatient bleeding or outpatient visits with major or minor bleeding. Major bleeding was defined as bleed in an important organ (intra-cranial, intra-ocular, intra-articular, etc), bleeding requiring blood product transfusion, a drop of more than 20 g/L in hemoglobin, bleeding requiring a surgical intervention or permanent discontinuation of anticoagulation.
TE and bleeding complications were assessed from discharge records and clinic follow-up notes.
Statistical analysis
Patient population characteristics were reported using the appropriate descriptive statistics according to variables distributions.
The proportion of time spent within predefined INR ranges was assessed by linear interpolation, as the proportion of time spent within the INR range assuming that INR variation between two consecutive values is linear. Since the distribution of the data was not normal, the Krustall-Wallis test was used to compare proportion of time spent with target INR between subgroups. All statistical analyses were done using R Core Team (2012) [7].
The study was approved by the CHUM’s ethic committee.
| Results | ▴Top |
Study patients
Patients were recruited at the anticoagulant clinic of the Hopital Hotel-Dieu of the CHUM between June 2010 and December 2011. A total of 347 patient’s charts were reviewed and 110 did not meet the inclusions criteria: 85 had a pathology that differed from DVT or PE, and 25 had less then 3 INR values. Reasons for exclusions are detailed in Table 1. Mean age of our population was 61 years old (21 to 97 years old) with a slight majority of women (125/237, 53%). Ninety (38%) patients had a past medical history of DVT/PE and 88 patients (37%) were anemic at the time of the study. Twelve patients (5%) had a past medical history of bleeding and 22 (9%) were on a concomitant antiplatelet drug regimen while on Warfarin (Table 2).
 Click to view | Table 1. Causes of Exclusion From the Study (n = 110) |
 Click to view | Table 2. Baseline Characteristics of Patients (n = 237) |
Treatment and INR evaluation
The median duration of follow-up was 281 days (Interquartile range (IQR) 98 - 378) with a total number of INR values of 2,553 during the study period. Median proportion of time at target INR (between 2.0 and 3.0) was 68.9%, IQR (48.7-85.4%). This proportion increased to 91.2%, IQR (78.9-100%) of time spent with INR values between 1.7 and 3.3, and 98.7%, IQR (89.8-100%) between 1.5 and 3.5 (Fig. 2).
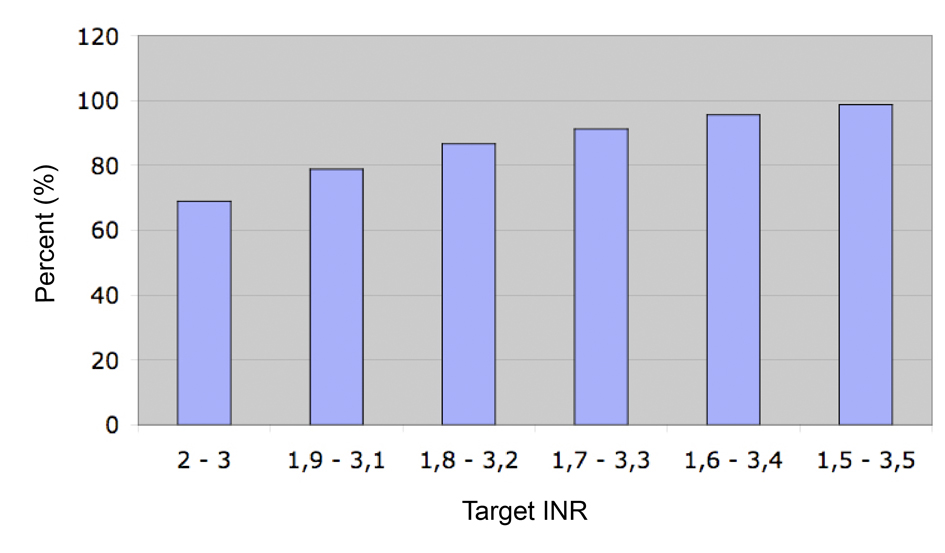 Click for large image | Figure 2. Median proportion of the time at target INR. |
Table 3 summarizes the median time spent within INR targets according to patient’s characteristics. Patients with chronic kidney disease were followed for longer period and had a median proportion of time at target INR of 80%, IQR (72-90%) as compared to 65%, IQR (48-83%) for patients without chronic kidney disease; p-value for difference is 0.048. There was a trend for an increased proportion of time spent within target INR with increasing age, but only few patients had a TE before the age of 40 years old (n = 25) and this did not reach statistical significance. There were no differences in time spent within INR target according to sex.
 Click to view | Table 3. Median Proportion of Time at Target INR Data According to Patient’s Characteristics (n = 237) |
Recurrent venous TE and hemorrhagic complications
There was a single recurrent TE event (Table 4). An 87 years old woman suffered a recurrent PE with an INR value of 2.1, representing a recurrence rate of 0.62 per 100 person-years (p-y), 95%CI (0.02 - 3.38). No underlying condition was identified to explain the recurrence.
 Click to view | Table 4. Thrombotic and Haemorrhagic Complications During Follow-Up (n = 17) |
There were 6 minor bleeding episodes (incidence rate 3.69 per 100 p-y, 95%CI (1.37 - 7.86)) and 10 majors bleeding episodes (incidence rate 6.15 per 100 p-y, 95%CI (2.99 - 11.02)) during our study period (Table 4). INR values were obtained in 3 of the 6 minor bleeding events (INR 2.0, 2.4 and 2.9) and all but one of the major bleeding events (INR 1.1, 1.4, 4.1, 2.6, 2.7, 3.8, 4.1, 6.3, and > 9.0). None of these episodes were intracranial bleeding or fatal. Two of the minor hemorrhagic events were epistaxis, one ENT related bleeding, one macroscopic hematuria, one hematochezia secondary to anal fissure and one iron-deficiency anemia secondary to menorrhagia. The majority of major bleeding episode were caused by gastrointestinal (GI) bleeding, with 5 episodes of lower GI bleeding and 2 of upper GI bleeding. The other hemorrhagic events were one post-traumatic leg hematoma, one knee hemarthrosis and one thigh hematoma that lead to hemodynamic instability and required transfusion of 3 packed red blood cell units. Overall, six of the major bleeding events required transfusion of blood product. Eight of the 10 major hemorrhagic episodes resulted in permanent termination of anticoagulation therapy.
| Discussion | ▴Top |
The purpose of this study was to review the performance of the internal medicine anticoagulation clinic at the Hotel-Dieu de Montreal of the CHUM. Our results show that patients spend a median of 68.9% of their time within therapeutical INR (between 2.0 and 3.0), and 98.7% of their time very close to therapeutical INR values (between 1.5 and 3.5) where bleeding and thrombosis risks are low. Surprisingly, we observed higher proportions of time spent with therapeutical INR values in patients with renal failure. This could be explained by closer follow-up of these patients. We also observed a trend for lower proportion of time spent at therapeutical INR values in younger patients, although our power was too limited to draw any conclusions, it is possible that younger patients tend to have looser follow-ups or were followed for a shorter period of time. Rates of symptomatic TE event were similar to results in the ELATE trial, but slightly lower than in most other studies and rates of bleeding events were similar to most studies [4, 5, 8-10].
We chose to report time spent within INR ranges that differ from the classical therapeutic target of 2.0 to 3.0. We believe this is well supported by the literature. The established therapeutic range of INR derives mainly from a randomised study published by Hull and all in 1982 [11, 12]. The purpose of this study was to determine if a “lower intensity anticoagulation” (INR between 2.0 and 2.3) compared to “moderate intensity anticoagulation” (INR between 2.5 and 4.1) would diminish the bleeding risk without reducing effectiveness for treatment of deep vein thrombosis (DVT). Other studies, done with the same purpose, showed a reduction in bleeding events while maintaining effectiveness for thrombosis prevention and treatment and systemic embolism in tissue heart valves and atrial fibrillation with less intense anticoagulation [13-16]. One of the earlier studies even showed effectiveness of anticoagulant therapy at range of INR between 1.6 and 2.5 for the treatment of acute myocardial infarction. This same study proved a significant risk reduction of 92% for strokes and 60% for pulmonary embolism (PE) (P < 0.05) [17]. Years later, the PREVENT Study showed that a target INR between 1.5 and 2.0 reduced the risk of recurrent TE events compared to placebo, but the ELATE trial reported slight superiority of higher INR target [18, 19]. It remains that a target INR between 1.5 - 2.0 is considered by some an effective and safe alternative for patients who need prolonged anticoagulant therapy but are at higher bleeding risk [20]. A review of hemorrhagic risk with anticoagulant therapy shows that exponential risk of bleeding and associated mortality are mostly present at INR values above 3.5 to 4.5 [21-23]. These results gave birth to the now famous INR u-shaped risk curve, showing the risk of thrombosis increases steeply at values lower than 1.5, and the risk of bleeding increases sharply at values higher than 5.0 [24]. Taken together, this literature suggests that INR values between 1.5 and 3.5 are relatively safe and clinically effective. While aiming at values between 2.0 and 3.0, clinicians can consider that values near limits of the range only need minor adjustments of posology and are not worrisome. It is therefore a very significant result to show that patients spend on average 98.7% of their time within this range.
Speaking about bleeding complications, we strongly believe that the majority of hemorrhage episodes in our study would be also seen with other anticoagulation drugs and were due to the patients’ bleeding risk and not to the drug of choice.
The retrospective and single-center design is limitation of this study. It is possible that the low rate of thrombotic and bleeding events is due to the retrospective design since events could have occurred at other centers, although the close follow-up done at the anticoagulation clinic makes this highly unlikely.
Strength of our study is the measurement method of our primary outcome (linear interpolation). This method gives a better representation of anticoagulation adequacy with Warfarin than the simple proportion of INR values falling within the therapeutic interval. Furthermore, reporting of time spend in values very close to the classical therapeutical target of 2.0 to 3.0 allows to grasp the rarity of extreme values when Warfarin is followed in a specialized anticoagulation clinic. Our study also has good external validity, since it was performed in a real-life setting, and patients with multiples co-morbidities were included in the study (old age, chronic kidney failure, past history of bleeding, concomitant use of antiplatelet therapy, etc.).
In conclusion, we demonstrated that patients followed in a specialized anticoagulation clinic spend on average 68.9% of their time within the therapeutical range of INR, but 98.7% of their time within very safe and effective INR values. Considering the multiple other advantages of Warfarin (observance measurement, availability of follow-up, low cost, and efficient proven antidote), we believe Warfarin retains a favorable profile compared to newer anticoagulant, especially for long term treatment. Data on observance to treatment of newer anticoagulants, which, outside the setting of randomized controlled trials, are given with very little follow-up, should be compared to the objective proof of adequate anticoagulation offered by Warfarin follow-up. To date, no real-life data can assure us that newer anticoagulant can provide long term, safe and effective anticoagulation.
Grant Support
None.
| References | ▴Top |
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675-683.
doi pubmed - Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102(2):268-278.
pubmed - Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ, 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761-768.
doi pubmed - Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510.
doi pubmed - Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297.
doi pubmed - Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
pubmed - A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria ISBN 3-900051-07-0.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
doi pubmed - Gitter MJ, Jaeger TM, Petterson TM, Gersh BJ, Silverstein MD. Bleeding and thromboembolism during anticoagulant therapy: a population-based study in Rochester, Minnesota. Mayo Clin Proc. 1995;70(8):725-733.
doi pubmed - Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinnon B, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631-639.
doi pubmed - Hull R, Hirsh J, Jay R, Carter C, England C, Gent M, Turpie AG, et al. Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med. 1982;307(27):1676-1681.
doi pubmed - Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324(26):1865-1875.
doi pubmed - Taberner DA, Poller L, Burslem RW, Jones JB. Oral anticoagulants controlled by the British comparative thromboplastin versus low-dose heparin in prophylaxis of deep vein thrombosis. Br Med J. 1978;1(6108):272-274.
doi pubmed - Francis CW, Marder VJ, Evarts CM, Yaukoolbodi S. Two-step warfarin therapy. Prevention of postoperative venous thrombosis without excessive bleeding. JAMA. 1983;249(3):374-378.
doi pubmed - Turpie AG, Gunstensen J, Hirsh J, Nelson H, Gent M. Randomised comparison of two intensities of oral anticoagulant therapy after tissue heart valve replacement. Lancet. 1988;1(8597):1242-1245.
doi - Bjerkelund CJ, Orning OM. The efficacy of anticoagulant therapy in preventing embolism related to D.C. electrical conversion of atrial fibrillation. Am J Cardiol. 1969;23(2):208-216.
doi - Assessment of short-anticoagulant administration after cardiac infarction. Report of the Working Party on Anticoagulant Therapy in Coronary Thrombosis to the Medical Research Council. Br Med J. 1969;1(5640):335-342.
doi pubmed - Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, Cushman M, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
doi pubmed - Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(22):2164-2167; author reply 2164-2167.
doi - Ridker PM. Long-term low-dose warfarin use is effective in the prevention of recurrent venous thromboembolism: yes. J Thromb Haemost. 2004;2(7):1034-1037.
doi pubmed - Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, Pengo V, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348(9025):423-428.
doi - Oden A, Fahlen M. Oral anticoagulation and risk of death: a medical record linkage study. BMJ. 2002;325(7372):1073-1075.
doi - Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):257S-298S.
- Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11-17.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


