| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 12, Number 2, April 2023, pages 59-65
Neutropenic Enterocolitis: An Uncommon, but Fearsome Complication of Leukemia
Rodrick Babakhanloua, c, Farhad Ravandi-Kashania, Dimitrios P. Kontoyiannisb
aDepartment of Leukemia, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
bDivision of Internal Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
cCorresponding Author: Rodrick Babakhanlou, Department of Leukemia, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
Manuscript submitted February 19, 2023, accepted April 29, 2023, published online April 30, 2023
Short title: Neutropenic Enterocolitis
doi: https://doi.org/10.14740/jh1105
| Abstract | ▴Top |
Neutropenic enterocolitis (NEC) is a life-threatening condition occurring in severely neutropenic patients, following intensive chemotherapy for leukemia. Its pathogenesis is not entirely understood and believed to be multifactorial, including mucosal injury as a result of cytotoxic drugs, profound neutropenia, impaired host defense and possibly microbiota changes. Establishing an early diagnosis is key. The management of NEC remains undefined due to lack of high-quality clinical data. With a better understanding of the disease, a more conservative approach is preferred over surgical intervention. The involvement of a multi-disciplinary team, consisting of the oncologist, infectious diseases specialists and surgeons is highly recommended. This review aims to delineate insights into the pathophysiology and clinical presentation of NEC and to emphasize the diagnostic and therapeutic approach to this condition.
Keywords: Neutropenic enterocolitis; Neutropenia; Leukemia
| Introduction | ▴Top |
The current approach to treating acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) incorporates the combination of potent chemotherapeutic agents and targeted molecular therapies with the goal of achieving complete remission (CR) or even cure [1-3]. Patients undergoing treatment for acute leukemia are at risk of life-threatening complications, either from the disease itself or from the therapy [3]. These complications can be either infectious or non-infectious, with a common involvement of the intestinal tract [4]. Non-infectious complications can occur as a result of cytotoxic chemotherapy in form of treatment-induced mucositis and can clinically present in form of nausea, vomiting, diarrhea or abdominal pain [5, 6]. Importantly, the severe and protracted neutropenia following chemotherapy places the patient at increased risk for a variety of infections [5, 7]. Neutropenia is defined as an absolute neutrophil count (ANC) of < 1,000/µL and is being classified as mild, moderate (< 500) and severe (< 100) [7-9]. The degree of neutropenia associated with chemotherapy should be expressed according to the Common Terminology Criteria for Adverse Events (CTCAE). In neutropenic patients, infections arising from the gastrointestinal tract (GIT) are common and can affect any part of the GIT. Indeed, in up to 30% of cases, the GIT is the source of infection in neutropenic patients, placing the patient at higher risk of subsequent sepsis, especially if the neutropenia is prolonged or severe [7, 10].
Due to the blunted response in neutropenic patients, the clinical presentation can be vague, making it challenging for the physician to differentiate between infectious and non-infectious causes of gastrointestinal (GI) complications [6, 11]. Therefore, it is crucial for the treating physician to both have a broad understanding of pathologic processes that can affect the GIT and a low threshold of suspicion to identify life-threatening conditions and to address those in a timely and effective fashion [7-12].
Pathologies affecting the GIT include neutropenic enterocolitis (NEC), Clostridium difficile colitis, cholecystitis and cholangitis, appendicitis, diverticulitis and perirectal cellulitis [4, 6, 8, 12]. Due to the broad spectrum of this topic, we herein focus only on NEC and discuss the pathophysiology, assessment and current controversies and challenges in its management.
| NEC | ▴Top |
NEC, also known as typhlitis, necrotizing enterocolitis or ileocecal syndrome, is a severe, frequently life-threatening complication seen in profoundly neutropenic patients and is typically manifested with the triad of fever, abdominal pain and diarrhea [13]. The term NEC was initially described as typhlitis, from the Greek word “typhlon”, meaning cecum. But since this condition can affect any part of the bowel, typhlitis is not all encompassing and has been replaced by NEC.
NEC is currently defined as neutropenia < 500 cells/L, fever > 38.3 °C and abdominal pain with abdominal computed tomography (CT) demonstrating > 4 mm bowel wall thickness in a > 3 cm length of bowel [7].
It was initially described in the pediatric population undergoing therapy for acute leukemia [13, 14]. However, over the years it was increasingly recognized in the adult population undergoing high-dose chemotherapy for both hematologic malignancies and solid tumors [13, 15-17]. NEC has also been rarely observed in patients with aplastic anemia, multiple myeloma and cyclic neutropenia [7, 12, 13, 18-22]. Although the cecum and the terminal ileum are most commonly involved, any part of the colon and the small intestine can be affected as well [6].
Its overall incidence is not known, as criteria are different among studies but it has been reported to occur in up to 32.5% of hospitalized patients undergoing chemotherapy for hematologic or solid malignancies and a mortality rate of at least 50% has been reported [7, 12, 18, 22, 23]. Other cause of variability in incidence is related to the patient population, type and intensity of chemotherapy used, comorbidities and differences in antibiotic/antifungal prophylaxis practices. NEC is in all likelihood clinically underestimated premortem as it was found in up to 46% of children with leukemia at autopsy [12, 19, 24]. It is unclear whether the routine antibacterial and antifungal prophylaxis results in a decreased incidence of NEC.
Risk factors
Patients with acute leukemia undergoing aggressive chemotherapy are commonly affected [23, 25]. Common cytotoxic drugs used in leukemia patients that induce mucosal injury and have been linked to the development of NEC include cytosine arabinoside (Ara-C), vincristine, doxorubicin or idarubicin [12]. Further risk factors include a prolonged duration of neutropenia, prior episodes of NEC and pre-existing bowel abnormalities, such as diverticular disease [24].
Pathogenesis
The pathogenesis of NEC is incompletely understood and probably multifactorial, due to the combination of mucosal injury, the presence of neutropenia and an impaired local and systemic host defense against intestinal microbiota [13, 19]. Mucosal injury typically is the result of remission induction of high-dose cytotoxic therapy and less commonly of mechanical lesions or leukemic infiltration of the bowel. In the setting of prolonged neutropenia, in addition to GIT and systemic infection, intramural hemorrhage can occur [13, 19]. Ara-C in particular is associated with mucosal alterations, acute mucosal injury and ulcerations and a necrosis of the mucosal layer, complicated by a delayed regeneration of mucosal epithelial cells [12]. In addition to its cytotoxicity to GIT mucosa, vincristine has been associated with damage of the autonomic ganglia, resulting in severe constipation and the development of a megacolon, increasing the risk of bowel ischemia and perforation [12]. Subsequently, the disruption of the mucosa results in intramural invasion of the bowel wall with bacteria, fungi or viruses, leading to a bowel wall edema, production of endotoxins and a cytotoxic edema with an increased risk of transmural necrosis [20, 22, 26]. As a complication of transmural ischemia and/or necrosis, bacterial or yeast translocation ensues, leading to subsequent bacteremia/candidemia, and in an immunocompromised host with neutropenia, consequently, to severe infection, even septic shock [19].
Various pathogens have been identified in surgical specimens, including gram-negative bacilli, gram-positive cocci, enterococci, anaerobes (e.g., clostridium septicum), Candida species and cytomegalovirus (CMV) [13]. The most common bacterial isolates were Pseudomonas spp., while Escherichia coli, Klebsiella spp., Staphylococcus aureus, and Streptococci were prevalent [13].
Candida species are the most common cause of fungal bloodstream infection in patients with NEC. While C. albicans, C. tropicalis, C. glabrata and C. krusei have been detected frequently, molds such as Aspergillus or Mucorales are frequently found in autopsy results as the cause of GIT infections but can be clinically silent [27]. In addition to classic features of NEC, intestinal bleeding in view of contiguous invasion of the gut wall might be a clue for fungal GIT invasion [28]. Other fungi, such as Histoplasma, Cryptococcus neoformans and Trichosporon spp. are less common [24, 29, 30].
In rare cases NEC has also been described in leukemic patients even before the administration of any chemotherapy. This has been believed to be due to potential leukemic infiltration of bowel wall or profound neutropenia or functional neutropenia [12, 13, 19].
Another important factor for the development of NEC is an alteration in the gut microbiome. The gut microbiome is an important factor for the maintenance of local (GIT) and systemic immune tone and immune homeostasis, mucosal integrity and competition that provides colonization resistance against invading pathogens. In cancer patients an alteration of the gut microbiome is frequently observed and can be the result from frequent and prolonged use of antibiotics, the impact of chemotherapy or diet modifications [19, 31].
A low diversity of both the oral and the gut microbiome has been frequently observed in leukemia patients [31]. Due to prolonged duration of neutropenia as a result of multiple lines of chemotherapy, it is not uncommon for patients to receive broad-spectrum antibiotics over a long period of time, which consequently results in a damage of the microbiome, promoting the growth of pathogenic bacteria [31]. It is unclear if specific microbiome compositions predispose neutropenic patients to the development of NEC.
The cecum is believed to be a very common site of involvement in NEC. As vascular supply of the cecum is less abundant compared to other parts of the bowel [12]. And since the cecum is more distensible than other parts of the bowel, it is believed that the distension of the cecum decreases the vascular perfusion, making it prone to mucosal breaches and subsequent ischemic necrosis [12].
However, a study that correlated CT imaging with histopathology and clinical information demonstrated that NEC was limited to the cecum in only 25% of cases, with more extensive colonic involvement in 75% of cases [32]. NEC can often extend into the terminal ileum or any parts of the small intestine [13].
Clinical presentation
Symptoms usually occur within 30 days of initiation of cytotoxic chemotherapy, often within 2 weeks following completion of therapy and coincide with the neutropenia nadir [19, 33]. Although the classic presentation of NEC includes profound neutropenia, fever > 38.3 °C, right-sided abdominal pain and diarrhea, patients can present with combinations of symptoms, such as abdominal distension, nausea, vomiting, bloody diarrhea or rebound tenderness in right lower abdomen, mimicking the clinical picture of acute appendicitis, bowel obstruction or infective colitis [13, 20, 29].
In early stages, patients may present with abdominal pain, fever and impaired bowel function [28]. Although the right lower quadrant is most commonly affected, the location of pain depends on the area of the affected bowel and can at times be diffuse [11, 33]. In severe cases additional symptoms, including abdominal cramping, nausea, diarrhea or even lower GI bleeding may be present [11-13, 24, 28, 32-34]. The presence of abdominal guarding, abdominal rigidity, absence of bowel signs, tachycardia, and tachypnea may indicate bowel perforation and the presence of sepsis [11]. Occasionally, paralytic ileus and bowel obstruction due to intestinal edema may occur, but these are rare [24].
Since cecal inflammation can be masked by the absence of neutrophils, a mass in the right lower quadrant can be the clinical sign on presentation, as shown in Figure 1 [30].
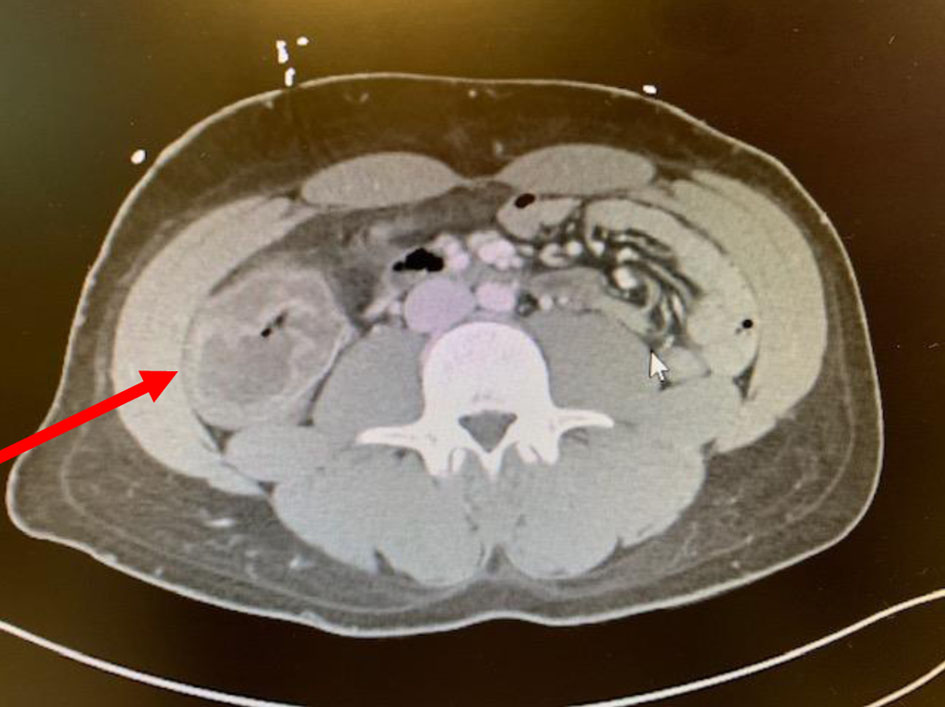 Click for large image | Figure 1. CT image of a 27-year-old man with relapsed and refractory AML with neutropenic enterocolitis, presenting with right lower quadrant mass. CT: computed tomography; AML: acute myeloid leukemia. |
It is important to keep in mind that associated septicemia is common, reported to occur in up to 73% of cases and that NEC can present with sepsis alone, even without any GI symptoms [12].
Although fever is present in most patients, it can be absent in severely immunocompromised patients, especially the ones taking glucocorticosteroids [24].
In certain circumstances, clinical signs may worsen, when patients recover from neutropenia and the restoration of the immune system causes an inflammatory response, characterized by abscess formation, bleeding or even perforation [24, 25].
Diagnosis
Establishing the diagnosis of NEC can be challenging, as there are not “gold standard” diagnostic criteria. Making the diagnosis is currently based on the combination of clinical picture, laboratory parameters and radiological findings [13, 19].
Although the clinical presentation of fever > 38.3 °C, right-sided abdominal pain and diarrhea in a neutropenic patient can be highly suggestive of NEC, it is still challenging to differentiate between infectious and non-infectious causes of abdominal pain based on those findings. Further clinical features can include signs of sepsis, GI emergencies, such as perforations, peritonitis or bleeding and the presence of abscesses [19]. Thus, the clinician needs to keep a broad spectrum of differential diagnosis in mind when approaching abdominal pain in neutropenic patients post chemotherapy, since clinical presentations like pain, nausea, diarrhea and vomiting are non-specific and may be caused by the toxic effects of chemotherapy, C. difficile colitis, ischemic colitis, infectious colitis, acute appendicitis or graft-versus-host disease (GVHD) in patients with prior allogeneic stem cell transplantation [7, 12, 18, 33]. The differential diagnosis of NEC is outlined in Table 1.
 Click to view | Table 1. Differential Diagnosis of Abdominal Pain in Neutropenic Patients [26] |
Laboratory evaluation should assess for electrolyte imbalances and pancytopenia, specifically investigating the degree of neutropenia [7, 19, 24]. Blood cultures, C. difficile toxin polymerase chain reaction (PCR) (and if positive, then toxin assay) and multiplex PCR testing of stool should be assessed in all patients [24].
The incidence of bacteremia ranges from 28% to 82% in different series [33]. Organisms isolated from blood cultures include aerobic gram-negative rods such as E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Enterobacter, gram-positive cocci such as Streptococci or even Candida species.
Radiographic imaging modalities are the most reliable diagnostic tool and include ultrasonography and CT [24]. Abdominal plain X-rays are of limited value in the diagnosis of NEC [24].
Although ultrasound is the preferred modality for the pediatric population due to the lack of ionizing radiation, CT is the preferred imaging modality in the adult population [13, 35]. This is because of the limited resolution of ultrasonography, especially in patients with certain body habitus types, its lower sensitivity and the operator dependence [24].
Characteristic sonographic patterns of NEC include a doughnut-like hypoechoic, fluid-filled intestinal lumen separated from thickened bowel wall by a thin hyperechoic line of mucosa [36]. However, despite its availability and low cost, ultrasound might suffer from intra-operator variability and low sensitivity in obese patients [36, 37].
CT can differentiate between various intrabdominal pathologies in neutropenic patients, and therefore, is the preferred option as it reveals the extend of GI involvement and the severity of GI wall inflammation [24]. Radiologic features include bowel wall thickening > 4 mm, pneumatosis intestinalis, bowel dilatation and mesenterial stranding as shown in Figures 1 and 2 [35]. The degree of bowel wall thickening significantly correlates with the outcome of patients [37]. In a retrospective study by Cartoni et al, 60% of patients with a bowel wall thickening of > 10 mm died from complications, compared to 4.2% in those with a bowel wall thickening of < 10 mm [37]. Other pertinent findings on CT include a fluid-filled dilated cecum, a right lower quadrant inflammatory mass or inflammatory changes affecting the perirectal soft tissues [20, 33].
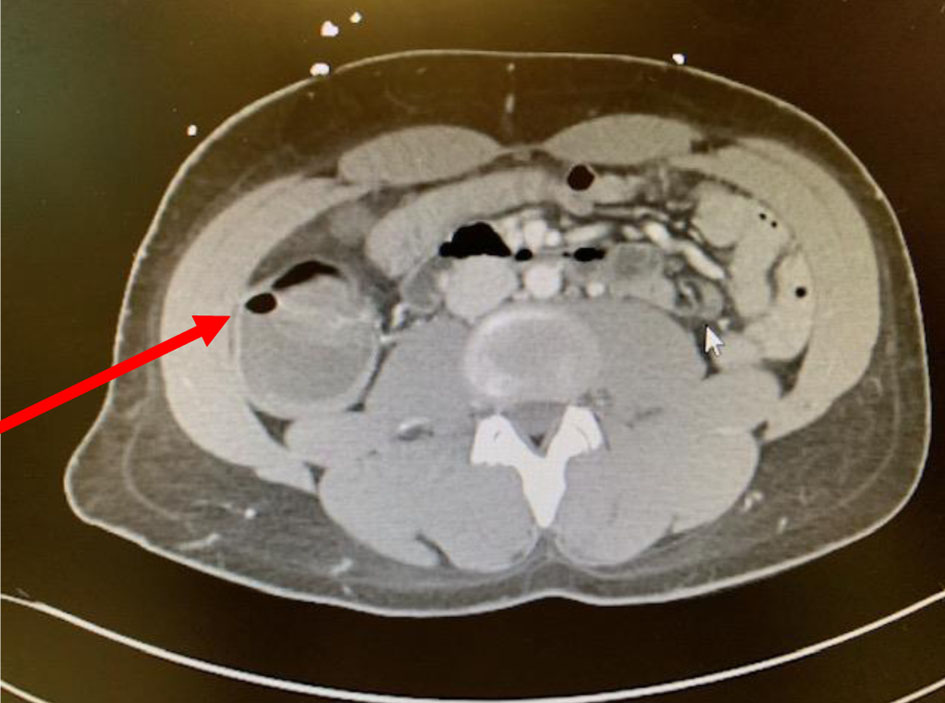 Click for large image | Figure 2. Sign of pneumatosis intestinalis in the same patient. |
Biopsies for confirmation of the diagnosis or for differentiation from CMV or GVHD colitis remain a challenge, since colonoscopies are contraindicated due to the risk of bowel perforation [26]. Moreover, there is an increased risk of focal or generalized bleeding in the affected areas of the GIT due to likely thrombocytopenia. If CMV colitis is suspected, qPCR for plasma CMV DNA as an adjunct diagnostic method for CMV GI disease has been reported to have an overall good sensitivity of 85% and a very good specificity of 95% [38]. Histopathological descriptions are mostly derived from autopsies which are reported in a minority of patients and this creates a selection bias as findings tend to overrepresent severe NEC. Typical post autopsy findings are hemorrhagic necrosis, patchy mucosal ulcerations, extensive edema in the submucosa and transmural necrosis. Importantly, significant infiltrative inflammation is absent in NEC due to profound neutropenia [19, 26, 37, 39].
Management
Due to lack of high-quality studies, it is difficult to make standardized recommendations on the approach of NEC [18, 33]. Treatment recommendations are based on descriptive studies, clinical experience or expert opinions [33].
Historically, initial reports preferred an aggressive surgical approach [7, 40]. But with a better understanding of the disease, treatment approaches have drifted away from aggressive surgical interventions towards more conservative modalities, and surgical management is certainly not the initial modality of treatment for NEC [7, 13, 20, 33].
In patients without complications, such as bleeding, perforation or peritonitis, non-surgical management, consisting of bowel rest, intravenous fluids, parenteral nutrition, blood product support and broad-spectrum antibiotic coverage, is a reasonable approach [13, 20, 33]. Importantly, an overuse of opioids and antiperistaltic agents should be limited, or ideally avoided, as they might impair the assessment of complications.
After having obtained blood cultures, the initial antimicrobial regimen should be empiric and target likely pathogens, including enteric gram-negative rods such as Pseudomonas aeruginosa and Escherichia coli, enterococci and anaerobes [24]. Antibiotic choice should be guided by institution-specific, and ideally, leukemia unit-specific antibiogram and take into considerations comorbidities, known colonization of patient’s GIT by pathogens (e.g., vancomycin-resistant enterococcal (VRE) colonization) and recent antibiotic exposures. Empiric coverage for C. difficile infection should be added if that diagnosis has not been excluded [33].
The time from triage to delivery of antibiotics should not exceed 1 h, as delays have been associated with decreased survival [41].
The IDSA guidelines recommend the prompt administration of empiric antibiotics and suggest cefepime, a carbapenem or piperacillin-tazobactam and recommend their use for at least the duration of neutropenia or until there are clear signs of marrow recovery [42]. However, those recommendations were made for the management of neutropenia and do not specifically address abdominal infections in neutropenic cancer patients or NEC specifically.
In cases of NEC where third generations cephalosporins are used as first agent, consideration of adding metronidazole for better coverage of anaerobes should be made. Addition of metronidazole should not be necessary if other lactams with excellent anaerobic coverage such as piperacillin/tazobactam or carbapenems are used. The role of empiric antifungal therapy upfront in NEC is debatable as bacterial causes are the commonest.
Since fungi have been reported to account for 53% of new microorganisms seen on autopsy and mortality has been reported to be up to 100% in patients with fungemia, antifungal therapy should be strongly considered in patients with persistent fever after 4 - 7 days of antibiotic therapy and in those, whose overall duration of neutropenia is expected to last longer than 7 days and should cover Candida spp. and Aspergillus [24, 30, 42]. Recommended options include voriconazole and amphotericin B formulations if mold coverage in addition to Candida is desired or echinocandins for candidemia arising from NEC.
Once the patient has been afebrile for at least 2 days and achieved an ANC > 500/cells, intravenous formulations can be deescalated to an appropriate oral regimen [42]. In patients with persisting mucositis, nausea and vomiting, intravenous formulations should be continued till recovery of GI symptoms and ability to tolerate oral intake.
The duration of treatment for NEC is not based on quality evidence and should be made on a case-to-case basis. We advocate at least 3 weeks of regimen and clinical reevaluation that includes repeat imaging with abdominal CT.
The use of granulocyte colony-stimulating factor (G-CSF) may be beneficial in severely ill patients with the intention to accelerate neutrophil recovery [24]. However, its use in NEC remains controversial, as it has not been adequately studied. Moreover, there are concerns of negatively impacting the integrity of the bowel wall in the setting of an augmented inflammatory reaction [24]. Hence, a routine use of G-CSF in patients with NEC is not recommended and needs to be evaluated on a case-to-case basis.
Although antibacterial and antifungal prophylaxis is standard of care in patients with acute leukemia receiving cytotoxic chemotherapy, NEC occurs, despite prophylactic antimicrobials.
Surgery should be avoided in the stable pancytopenic patients with NEC in view of the increased risk of postsurgical infections, impaired wound healing and an increased risk of bleeding [43]. Surgical intervention is suitable in cases of complications, such as bowel perforation, persistent GI bleeding or development of intraabdominal complications, such as abscesses or necrosis [40]. In emergency surgeries, such as in perforation, a two-stage procedure is preferred over a primary anastomosis [12].
Not surprisingly, urgent operations, in order to repair hollow viscus perforations in the setting of severe neutropenia and thrombocytopenia are significantly associated with increased mortality and morbidity rates of 11.8% and 54.8%, respectively [43].
| Conclusion | ▴Top |
NEC is a life-threatening condition occurring in severely neutropenic patients, following intensive chemotherapy, especially for patients with acute leukemia. Its pathogenesis is not entirely understood, and it is multifactorial. Establishing an early diagnosis is key and the role of early abdominal CT is important. The management of NEC remains undefined due to lack of high-quality clinical data. With a better understanding of the disease, a more conservative approach is preferred over surgical intervention. A general approach should be individualized based on the severity of gut involvement (extend and depth of involvement), pre-existing comorbidities and risk factors for severe NEC, and the presence of complications and should include a multi-disciplinary team, consisting of the oncologist, infectious diseases specialists and surgeons.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
RB has no conflict of interest. FR receives honoraria, research funds, consulting fees from Astellas, AstraZeneca, Xenocor, Prelude, Abbvie, Novartis, Syos, Amgen, Celgene/BMS and Astex/Taiho. DPK reports honoraria and research support from Gilead Sciences and Astellas, Inc., received consultant fees from Astellas Pharma, Merck, and Gilead Sciences, and is a member of the Data Review Committee of Cidara Therapeutics, AbbVie, and the Mycoses Study Group.
Author Contributions
RB wrote the manuscript. FR revised and approved manuscript. DK corrected, updated and approved manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; ANC: absolute neutrophil count; CR: complete remission; CT: computed tomography; G-CSF: granulocyte colony-stimulating factor; GIT: gastrointestinal tract; NEC: neutropenic enterocolitis
| References | ▴Top |
- Babakhanlou R, Ravandi-Kashani F. SOHO State of the Art Updates and Next Questions. The role of maintenance therapy in acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2023;23(1):1-7.
doi pubmed - Babakhanlou R, Ravandi-Kashani F. Non-intensive acute myeloid leukemia therapies for older patients. Expert Rev Hematol. 2023;16(3):171-180.
doi pubmed - Carobolante F, Chiaretti S, Skert C, Bassan R. Practical guidance for the management of acute lymphoblastic leukemia in the adolescent and young adult population. Ther Adv Hematol. 2020;11:2040620720903531.
doi pubmed pmc - Lebon D, Biard L, Buyse S, Schnell D, Lengline E, Roussel C, Gornet JM, et al. Gastrointestinal emergencies in critically ill cancer patients. J Crit Care. 2017;40:69-75.
doi pubmed - Leach C. Complications of systemic anti-cancer therapy. Medicine. 2022;50(12):805-808.
- Gorschluter M, Glasmacher A, Hahn C, Leutner C, Marklein G, Remig J, Schmidt-Wolf IG, et al. Severe abdominal infections in neutropenic patients. Cancer Invest. 2001;19(7):669-677.
doi pubmed - White MG, Morgan RB, Drazer MW, Eng OS. Gastrointestinal surgical emergencies in the neutropenic immunocompromised patient. J Gastrointest Surg. 2021;25(12):3258-3264.
doi pubmed pmc - Siebert M, Lucas N, Gelli M, Sourrouille I, Benhaim L, Faron M, Micol JB, et al. Acute abdominal complications in deeply neutropenic onco-hematology patients: a retrospective series of 105 cases. World J Surg. 2022;46(10):2389-2398.
doi pubmed - Abu-Sbeih H, Ali FS, Chen E, Mallepally N, Luo W, Lu Y, Foo WC, et al. Neutropenic enterocolitis: clinical features and outcomes. Dis Colon Rectum. 2020;63(3):381-388.
doi pubmed - Perazzoli C, Feitosa MR, Lobo de Figueiredo-Pontes L, et al. Management of acute colorectal diseases in febrile neutropenic patients. Journal of Coloproctology. 2014;34(3):189-192.
- Babakhanlou R. Upper abdominal pain. InnovAiT. 2018;11(8):428-434.
- Ebert EC, Hagspiel KD. Gastrointestinal manifestations of leukemia. J Gastroenterol Hepatol. 2012;27(3):458-463.
doi pubmed - Davila ML. Neutropenic enterocolitis. Curr Opin Gastroenterol. 2006;22(1):44-47.
doi pubmed - Wagner ML, Rosenberg HS, Fernbach DJ, Singleton EB. Typhlitis: a complication of leukemia in childhood. Am J Roentgenol Radium Ther Nucl Med. 1970;109(2):341-350.
doi pubmed - Sullivan PS, Moreno C, Shaib WL. Management of anorectal and intra-abdominal infections in the neutropenic cancer patient. Curr Probl Cancer. 2015;39(5):274-286.
doi pubmed - Hogan WJ, Letendre L, Litzow MR, Tefferi A, Hoagland HC, Pruthi RK, Kaufmann SH. Neutropenic colitis after treatment of acute myelogenous leukemia with idarubicin and cytosine arabinoside. Mayo Clin Proc. 2002;77(8):760-762.
doi pubmed - Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Neutropenic enterocolitis in an advanced epithelial ovarian cancer patient treated with paclitaxel/platinum-based chemotherapy: a case report and review of the literature. Anticancer Res. 2005;25(3c):2509-2513.
pubmed - Gorschluter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG, Sauerbruch T, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75(1):1-13.
doi pubmed - Kapandji N, Azoulay E, Zafrani L. Recent advances in neutropenic enterocolitis: Insights into the role of gut microbiota. Blood Rev. 2022;54:100944.
doi pubmed - Davila M, Bresalier RS. Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol. 2008;5(12):682-696.
doi pubmed - Aksoy DY, Tanriover MD, Uzun O, Zarakolu P, Ercis S, Erguven S, Oto A, et al. Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis. Ann Oncol. 2007;18(1):183-189.
doi pubmed - Hordonneau C, Montoriol PF, Guieze R, Garcier JM, Da Ines D. Abdominal complications following neutropenia and haematopoietic stem cell transplantation: CT findings. Clin Radiol. 2013;68(6):620-626.
doi pubmed - Gorschluter M, Marklein G, Hofling K, Clarenbach R, Baumgartner S, Hahn C, Ziske C, et al. Abdominal infections in patients with acute leukaemia: a prospective study applying ultrasonography and microbiology. Br J Haematol. 2002;117(2):351-358.
doi pubmed - Nesher L, Rolston KV. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin Infect Dis. 2013;56(5):711-717.
doi pubmed - Badgwell BD, Cormier JN, Wray CJ, Borthakur G, Qiao W, Rolston KV, Pollock RE. Challenges in surgical management of abdominal pain in the neutropenic cancer patient. Ann Surg. 2008;248(1):104-109.
doi pubmed - Xia R, Zhang X. Neutropenic enterocolitis: A clinico-pathological review. World J Gastrointest Pathophysiol. 2019;10(3):36-41.
doi pubmed pmc - Lewis RE, Cahyame-Zuniga L, Leventakos K, Chamilos G, Ben-Ami R, Tamboli P, Tarrand J, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56(6):638-645.
doi pubmed - Kontoyiannis DP, Mathur M, Chen YB, Shellito PC, Tse JY. Case records of the Massachusetts General Hospital. Case 13-2014. A 41-year-old man with fever and abdominal pain after stem-cell transplantation. N Engl J Med. 2014;370(17):1637-1646.
doi pubmed - Granwehr B, Kontoyiannis DP. The evolving landscape of gastrointestinal infections in neutropenic patients. Oncology (Williston Park). 2015;29(8):590, 592-593.
pubmed - Gorschluter M, Mey U, Strehl J, Schmitz V, Rabe C, Pauls K, Ziske C, et al. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35.
doi pubmed pmc - Galloway-Pena JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, Chemaly RF, et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122(14):2186-2196.
doi pubmed pmc - Babakhanlou R. Lower gastrointestinal bleeding. InnovAiT. 2018;11(3):138-142.
- Davila ML. Neutropenic enterocolitis: current issues in diagnosis and management. Curr Infect Dis Rep. 2007;9(2):116-120.
doi pubmed - Bavaro MF. Neutropenic enterocolitis. Curr Gastroenterol Rep. 2002;4(4):297-301.
doi pubmed - Kirkpatrick ID, Greenberg HM. Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology. 2003;226(3):668-674.
doi pubmed - Ullery BW, Pieracci FM, Rodney JR, Barie PS. Neutropenic enterocolitis. Surg Infect (Larchmt). 2009;10(3):307-314.
doi pubmed - Cartoni C, Dragoni F, Micozzi A, Pescarmona E, Mecarocci S, Chirletti P, Petti MC, et al. Neutropenic enterocolitis in patients with acute leukemia: prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19(3):756-761.
doi pubmed - Durand CM, Marr KA, Arnold CA, Tang L, Durand DJ, Avery RK, Valsamakis A, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis. 2013;57(11):1550-1559.
doi pubmed pmc - Sachak T, Arnold MA, Naini BV, Graham RP, Shah SS, Cruise M, Park JY, et al. Neutropenic enterocolitis: new insights into a deadly entity. Am J Surg Pathol. 2015;39(12):1635-1642.
doi pubmed - Shamberger RC, Weinstein HJ, Delorey MJ, Levey RH. The medical and surgical management of typhlitis in children with acute nonlymphocytic (myelogenous) leukemia. Cancer. 1986;57(3):603-609.
doi pubmed - Braga CC, Taplitz RA, Flowers CR. Clinical implications of febrile neutropenia guidelines in the cancer patient population. J Oncol Pract. 2019;15(1):25-26.
doi pubmed pmc - Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad, II, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-93.
doi pubmed - Jolissaint JS, Harary M, Saadat LV, Madenci AL, Dieffenbach BV, Al Natour RH, Tavakkoli A. Timing and outcomes of abdominal surgery in neutropenic patients. J Gastrointest Surg. 2019;23(4):643-650.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


