| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 3, June 2023, pages 133-137
Treating Acquired Factor VIII Inhibitor and Tumor-Induced Hypoglycemia in a Case of Relapsed Diffuse Large B-Cell Lymphoma
Preston Bakera, Joseph Nortona, c, Nasheed Hossainb
aStritch School of Medicine, Loyola University Chicago, Maywood, IL, USA
bPerelman School of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
cCorresponding Author: Joseph Norton, Stritch School of Medicine, Loyola University Chicago, Maywood, IL, USA
Manuscript submitted March 23, 2023, accepted May 17, 2023, published online June 30, 2023
Short title: Treatment of FVIII Inhibitor and TIH in RR-DLBCL
doi: https://doi.org/10.14740/jh1118
| Abstract | ▴Top |
Diffuse large B-cell lymphoma (DLCBL) is a heterogenous disease, with many phenotypic subtypes and occasional paraneoplastic syndromes being present. Herein, we describe a case of a 63-year-old woman, with relapsed/refractory DLBCL (RR-DLBCL) with artifactual hypoglycemia on laboratory testing, likely related to the mechanical effects of a new factor VIII inhibitor. We demonstrate our workup, consideration, treatment, and her clinical course. This patient did not present with a bleeding phenotype despite her aberrant laboratory results, and therefore determining her risk of bleeding to weigh against further diagnostic procedures presented a difficult decision. We utilized rotational thromboelastometry (ROTEM) to assist with clinical decision making regarding her paraneoplastic factor VIII inhibitor and the patient’s bleeding risk. This led to a short course of dexamethasone. Her ROTEM improved, and an excisional biopsy was performed without any bleeding. To our knowledge, this is the only reported instance where this technology was utilized in this setting. We believe utilizing ROTEM to determine bleeding risk may be a beneficial tool for clinical practice in such additional rare cases.
Keywords: Thromboelastogram; ROTEM; DLBCL; Factor VIII; Inhibitor; TIH
| Introduction | ▴Top |
Non-Hodgkin lymphoma (NHL) characterizes a heterogenous group of lymphoid malignancies with varied etiologies, presentations, and natural histories. Diffuse large B-cell lymphoma (DLBCL) makes up the most common subtype of NHL and has long been one of the most aggressive malignancies with high rates of mortality [1, 2]. This high mortality is primarily driven by cases that are not responsive to first-line therapy, known as either primary refractory if complete remission (CR) is not achieved with induction chemotherapy, or relapsed remitting DLBCL if relapse occurs within 6 months of achieving CR [2-5]. Therefore, adequate differentiation between the NHLs and staging are paramount to provide the most appropriate trade-off between treatment toxicity and benefit [6]. Complicating this, NHLs are well known to cause various paraneoplastic syndromes, ranging from neurologic to rheumatologic, and these may delay appropriate care [7, 8]. Herein we will discuss an unusual case of relapsed/refractory DLBCL (RR-DLBCL) with a rare presentation of a rare paraneoplastic syndrome at the time of admission for induction chemotherapy, detailing the workup, treatment, and outcomes.
| Case Report | ▴Top |
Investigations
Patient history and initial presentation
A 63-year-old woman with a history of stage IV marginal-zone lymphoma with large cell transformation with CR following one cycle of rituximab, cyclophosphamide, vincristine sulfate, and prednisone, and six cycles of cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine, was admitted to the hospital for workup of a painful abdominal mass identified on surveillance computed tomography. At the time of presentation, the patient had constitutional B-symptoms including unintentional weight loss, lightheadedness, and severe fatigue concerning disease relapse. Computed tomography scan showed diffuse lymphadenopathy present throughout the neck, chest, abdomen, and pelvis, and hepatosplenomegaly. Plan was made for exploratory laparotomy to obtain an excisional biopsy to confirm the diagnosis of RR-DLBCL. Routine preoperative studies were significant for severe hypoglycemia, normal anion gap metabolic acidosis, and a significantly elevated activated partial thromboplastin time (aPTT) (Table 1). Other than a lacy, reticular rash that was first noticed by the patient a few days before admission, there were no physical exam findings present to endorse the presence of a coagulopathic phenotype. The patient denied any history of clinically significant bleeding episodes, but did have a history of a provoked, superficial lower extremity deep vein thrombus over 20 years prior. The patient also denied any symptoms of hypoglycemia including new onset fatigue, lightheadedness, confusion, palpitations, or tremor. She was started on an intravenous (IV) dextrose drip with improvement of her serum glucose to the 80 - 100 s (Table 1).
 Click to view | Table 1. Preoperative Labs Ordered for Inpatient Workup of Relapsed Lymphoma |
Workup of coagulopathy
Factor levels and mixing studies as well as lupus anticoagulant studies were obtained to work up for any acquired deficiency or antibody explaining the elevated aPTT (Table 2). The rotational thromboelastometry (ROTEM) was performed to confirm the presence of coagulopathy. Rationale for utilization of ROTEM was based on the institution’s availability, the rapidity of results of testing, and its known utility in the perioperative setting. Results of ROTEM were consistent with the serologic studies that had been performed prior that confirmed the patient’s high risk of bleeding, with a severely prolonged clotting time and weak clot strength (Fig. 1a).
 Click to view | Table 2. Further Workup of Coagulopathy and Hyperglycemia Identified at Admit |
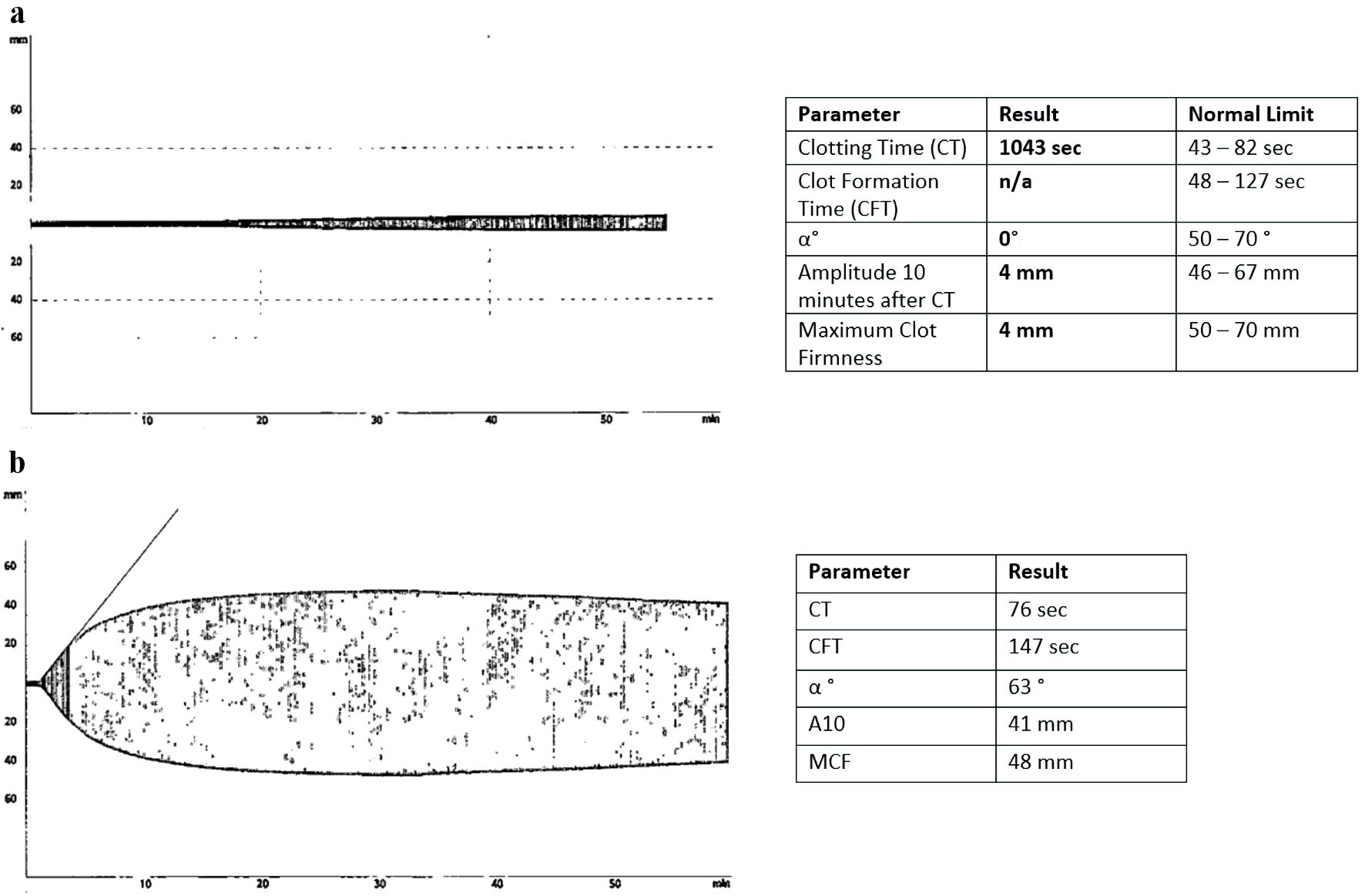 Click for large image | Figure 1. Rotational thromboelastometry (ROTEM) profile of the patient before and after treatment with pulse dose steroids. (a) The ROTEM results at the time of admission. Patient had a severely prolonged clotting time (CT) at 1,043 s, an immeasurably long clot formation time (CFT), consistent with severe coagulopathy. Due to the patient’s immeasurable CFT, the alpha angle (α) could not be measured. (b) The ROTEM results following a 4-day course of high-dose dexamethasone with normalization of CT and the CFT, and α angle normalized as well, consistent with a strong clot strength. Of note, the patient’s maximum lysis time was significantly reduced indicating defective fibrinolysis even after the course of dexamethasone. |
Workup of hypoglycemia
Endogenous causes of hypoglycemia were ruled out including insulinoma, adrenal insufficiency, and tumor-induced hypoglycemia (TIH) (Table 2). Workup for an insulinoma, pituitary insufficiency, and iatrogenic hypoglycemia was negative, increasing concern for TIH secondary to refractory DLBCL. The decision was also made to simultaneously draw capillary glucose readings with each basic metabolic panel (BMP), which showed discordance between the two values. This was interpreted as confirmation that the falsely decreased serum glucose levels on BMP’s, was at least partially a laboratory artifact likely derived from the mechanical effects of her newly acquired monoclonal paraprotein, which is a known entity in other diseases such as Waldenstrom’s macroglobulinemia.
Treatment and outcomes
Treatment of coagulopathy
On hospital day 10, the presence of an inhibitor to factor VIII was confirmed on repeat testing. The decision was made to treat the patient with a 4-day high-dose dexamethasone pulse to rapidly reduce the B-cell clone producing the factor VIII inhibitor and decrease factor VIII clearance. Follow-up factor inhibitor levels showed a small change in the concentration of the inhibitor (Table 3), but repeat ROTEM testing on hospital day 14 showed only mild delayed clotting time as well as mild clot fibrinolysis, a significant improvement from prior (Fig. 1b).
 Click to view | Table 3. Coagulation Studies and Factor VIII Levels Over Time |
Workup and treatment of relapsed DLBCL
Initially, the decision was made to defer exploratory laparotomy for diagnosis due to persistent hypoglycemia and concern for a possible acquired coagulopathy. Anticoagulation was held, and a bone marrow biopsy was obtained showing lymphoplasmacytic lymphoma. No signs or symptoms of bleeding occurred following the procedure. After the dexamethasone course, the patient’s ROTEM improved, showing only an insignificant coagulopathy. Combined with the patient’s consistent lack of bleeding, the benefits of lymph node biopsy in guiding disease management were deemed to outweigh the risks. A cervical deep lymph node biopsy was performed without complications, and the diagnosis of DLBCL was confirmed. The patient was treated with a salvage chemotherapy regimen of rituximab, ifosfamide, carboplatin, and etoposide. Following treatment labs demonstrated resolution of the patient’s acquired hemophilia (Table 3), although ROTEM was not performed again.
| Discussion | ▴Top |
Herein, we described a case of RR-DLBCL, presenting with artifactual hypoglycemia, and paraneoplastic acquired hemophilia, representing the disseminated and aggressive nature of NHL. To the best of our knowledge, this is the first reported case of DLBCL in which the patient had lab evidence of hypoglycemia and an acquired coagulopathy.
Acquired factor inhibitors and the development of new lupus anticoagulant antibodies are rare but known phenomena in lymphoproliferative disorders [9-14]. This paraneoplastic syndrome is frequently reported as a presenting symptom in recurrent or rare lymphomas that are frequently aggressive in nature. It is presumed that the acquired coagulopathy resolves with treatment of the underlying malignancy [10]. As seen in this case, tumors can produce a heterogenous profile of antibodies towards the coagulation cascade, varying in both serum concentration and binding profile. This leads to substantial variability in the observed bleeding risk from patient to patient. Lab abnormalities can even be false positives, with one case report confirming the values erroneous with further testing [15]. Although further research is needed to validate ROTEM as an optimal tool in assessing an individual’s coagulopathic phenotype, its application could be of significant use to clinicians in assessing bleeding and clotting risk in patients with factor inhibitors.
TIH is a life-threatening complication of many non-islet cell malignancies, including lymphomas [16-22]. It is likely that the presence of hypoglycemia typically represents advanced disease, and urgent treatment is necessary; however, our case represents an alternate presentation where the abnormality was likely artifactual due to laboratory processing. This is a rare but reported finding typically associated with elevated M protein in multiple myeloma or lymphoplasmacytic lymphoma [23]. In these cases, point-of-care glucose monitoring also demonstrated the discordant results and presented a simple solution for the clinician to determine an accurate glucose [23].
Excisional lymph node biopsy is a critical component of management of lymphoma, which is highlighted in this case where the bone marrow had discordant pathology from the lymph node [3, 24]. The patient’s complications described above represent barriers that could have prolonged the initiation of optimal therapy and exposed the patient to unnecessary therapies such as hypotonic fluids to correct hypoglycemia, and blood products to correct coagulopathy. Triaging abnormalities such as factor VIII inhibitors may interrupt adequate workup, and ROTEM may be an invaluable tool going forward.
Acknowledgments
None to declare.
Financial Disclosure
We received no funding and have no financial conflict to disclose.
Conflict of Interest
We have no conflict of interest to disclose.
Informed Consent
Patient consented to the publishment of this case.
Author Contributions
PB: writing and literature search. JN: writing, literature search, and editing. NH: concept and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842-858.
doi pubmed pmc - Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96(5):617-629.
doi pubmed pmc - Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, Connors JM, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29(11):1452-1457.
doi pubmed - Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, Rubinger M, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490-3496.
doi pubmed - Wang Y, Farooq U, Link BK, Larson MC, King RL, Maurer MJ, Allmer C, et al. Late relapses in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2019;37(21):1819-1827.
doi pubmed pmc - Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
doi pubmed - Guha A, Chaurasia K, Nath BD, Basu R, Das A, Choudhury D, Joshi M, et al. Paraneoplastic diseases associated with non-Hodgkin’s lymphoma - case series. Eastern Journal of Medical Sciences. 2020;5(3):66-69.
doi - Bossard V, Sauvageon Y, Fraissinet F, Dreyfus B, Thuillier R, Hauet T. [False paraprotein-induced hypoglycemia in the measurement of glucose by the hexokinase method]. Ann Biol Clin (Paris). 2019;77(4):439-445.
doi pubmed - Naismith K, Allevato PA, Hamm C. A rare case of factor VII inhibitor in a patient presenting with primary splenic marginal zone lymphoma. Am J Case Rep. 2021;22:e932704.
doi pubmed pmc - Mariotti J, Locatelli G, Cirrincione S, Agostinelli E, Corti D, Maggioni A, Falanga A, et al. Eradication of acquired hemophilia associated with indolent non-Hodgkin lymphoma by a disease specific treatment. Leuk Lymphoma. 2015;56(11):3210-3212.
doi pubmed - Lechner K, Simonitsch I, Haselbock J, Jager U, Pabinger I. Acquired immune-mediated thrombophilia in lymphoproliferative disorders. Leuk Lymphoma. 2011;52(10):1836-1843.
doi pubmed - Ediriwickrema LS, Zaheer W. Diffuse large cell lymphoma presenting as a sacral mass and lupus anticoagulant. Yale J Biol Med. 2011;84(4):433-438.
pubmed pmc - Nixon CP, Prsic EH, Guertin CA, Stevenson RL, Sweeney JD. Acquired factor XIII inhibitor associated with mantle cell lymphoma. Transfusion. 2017;57(3):694-699.
doi pubmed - Rochanda L, Del Zoppo GJ, Feinstein DI, Liebman HA. Approach to the treatment, characterization and diagnosis of an acquired auto-antibody directed against factors prothrombin, factor X and factor IX: a case report and review of the literature. Haemophilia. 2012;18(1):102-107.
doi pubmed - Sadatani K, Niiya K, Sonobe H, Sasaki K, Miyamoto I, Nakano M, Habara T, et al. Prolonged activated partial thromboplastin time and false-positive results for fibrinogen and fibrin degradation products in a B-cell lymphoma patient. Ann Clin Lab Sci. 2018;48(3):377-380.
pubmed - Durig J, Fiedler W, de Wit M, Steffen M, Hossfeld DK. Lactic acidosis and hypoglycemia in a patient with high-grade non-Hodgkin's lymphoma and elevated circulating TNF-alpha. Ann Hematol. 1996;72(2):97-99.
doi pubmed - Di Comite G, Dagna L, Piatti PM, Monti LD, Tantardini F, Praderio L. Hypoglycaemia and lactic acidosis in a MALT non Hodgkin's lymphoma. Leuk Lymphoma. 2002;43(6):1341-1342.
doi pubmed - Lopez Rodriguez M, Vazquez Munoz E, Gomez Cerezo J, Pagan Munoz B, Ruiz Bravo-Burguillos E, Barbado Hernandez FJ. [Lactic acidosis, severe hypoglycemia and hepatosplenomegaly]. Rev Clin Esp. 2007;207(10):521-522.
doi pubmed - Kahl C, Jost K, Knauerhase A, Leithauser M, Freund M, Bunke D, Rothacker D, et al. [Recurrent hypoglycemia and a large intraabdominal tumor in a 61-year-old woman]. Dtsch Med Wochenschr. 2011;136(49):2542-2546.
doi pubmed - Elhomsy GC, Eranki V, Albert SG, Fesler MJ, Parker SM, Michael AG, Griffing GT. "Hyper-warburgism," a cause of asymptomatic hypoglycemia with lactic acidosis in a patient with non-Hodgkin's lymphoma. J Clin Endocrinol Metab. 2012;97(12):4311-4316.
doi pubmed - Talwalkar PG. Severe and persistent hypoglycemia with lactic acidosis in an elderly lady with type 2 diabetes mellitus and lymphoma\leukemia: A rare case report. Diabetes Metab Syndr. 2019;13(1):648-650.
doi pubmed - Vega-Cano S, Cordero-Vazquez E, Mestre-Torres J. Hypoglycemia as an onset form of hypophysial infiltration by lymphoma. Med Clin (Barc). 2021;156(7):362-363.
doi pubmed - Wenk RE, Yoho S, Bengzon A. Pseudohypoglycemia with monoclonal immunoglobulin m. Arch Pathol Lab Med. 2005;129(4):454-455.
doi pubmed - Laurent C, Baron M, Amara N, Haioun C, Dandoit M, Maynadie M, Parrens M, et al. Impact of expert pathologic review of lymphoma diagnosis: study of patients from the french lymphopath network. J Clin Oncol. 2017;35(18):2008-2017.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


