| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 5, October 2023, pages 201-207
The Impact of Renal Impairment in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation With Melphalan Conditioning
Sorana G. Ursua, b , Samantha Maplesa, Kiersten J. Williamsa, Gina Patrusa, Yazan Samhouria, Salman Fazala, Prerna Mewawallaa, Santhosh Sadashiva
aDivision of Hematology/Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA 15224, USA
bCorresponding Author: Sorana G. Ursu, Division of Hematology/Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA 15224, USA
Manuscript submitted July 11, 2023, accepted September 13, 2023, published online October 21, 2023
Short title: Renal Impairment and Melphalan Conditioning
doi: https://doi.org/10.14740/jh1148
| Abstract | ▴Top |
Background: There are no standard renal dose adjustments for melphalan conditioning for autologous stem cell transplantation (ASCT) in multiple myeloma (MM) patients. The objective of this study was to evaluate the effect of melphalan dosing and chronic kidney disease (CKD) on transplant-related outcomes, progression-free survival (PFS), and overall survival (OS).
Methods: A retrospective chart review was performed, and MM patients who underwent ASCT between February 2016 and September 2021 were included. Melphalan 200 mg/m2 (Mel200) or 140 mg/m2 (Mel140) was administered. The cohort was divided based on renal function: creatinine clearance (CrCl) ≥ 60 mL/min (no-CKD) and CrCl < 60 mL/min (CKD). Outcomes measured include PFS, OS, treatment-related mortality (TRM), incidence of adverse events, hospitalization duration, and hospital readmission within 30 days. Statistical analysis included Chi-square test, t-test, and Kaplan-Meier method. Logistic regression model was used to account for melphalan dose adjustment.
Results: A total of 124 patients were included (n = 108 no-CKD, and n = 16 CKD). Median age was 62 years, majority (62%) were male, and 97% had at least a partial response at time of ASCT. Of the 124 patients, nine (7%) received Mel140. Five of these patients had CKD (CrCl range: 26 - 58 mL/min), with one on hemodialysis. Median time to neutrophil engraftment was 13.6 vs. 14.9 days and median time to platelet engraftment was 18.3 vs. 18.5 days in the CKD group vs. no-CKD group, respectively (P = 0.03 and P = 0.8). When adjusting for melphalan dose reduction, the median time to neutrophil engraftment was not statistically significant (P = 0.11). At a median follow-up of 28.7 months, the median PFS for the CKD vs. no-CKD group was 60 vs. 46 months (P = 0.3). One-year OS was 93.8% in the CKD group vs. 97% in the no-CKD group. There was a higher incidence of grade 3 or 4 mucositis in the CKD group vs. no-CKD group (P = 0.013).
Conclusions: There is no significant difference in engraftment, PFS, or OS for MM patients with CKD vs. no-CKD receiving melphalan conditioning for ASCT. Severe mucositis was significantly more common in the CKD group, including when accounting for melphalan dose reduction.
Keywords: Renal impairment; Melphalan; Autologous stem cell transplantation
| Introduction | ▴Top |
Melphalan has been a staple in cancer treatment and hematopoietic stem cell transplant for over 60 years [1]. Despite its longevity and wide array of uses over time, there are still no standard melphalan renal dose adjustments, particularly when used in autologous stem cell transplantation (ASCT) for multiple myeloma (MM) [2]. Although melphalan is primarily metabolized by the liver and through chemical hydrolysis, renal impairment can increase melphalan serum concentration [3]. The extent and predictability of this increase is not well defined, and the clinical consequences on treatment-related mortality (TRM), progression-free survival (PFS), and treatment-related toxicities are unknown. Prior studies examining this relationship have shown variable creatinine clearance (CrCl) cutoffs and mixed results on the safety and efficacy of melphalan conditioning for ASCT in MM patients with renal dysfunction; however, there appears to be a trend towards increased toxicity and little impact on overall survival (OS) [4-6]. Although there have not been significant correlations between melphalan dose in renal dysfunction and OS, there are studies showing improved treatment response with higher melphalan doses in patients with renal dysfunction [7, 8]. We aimed to evaluate the impact of renal dysfunction on the safety and efficacy of melphalan conditioning chemotherapy for ASCT in patients with MM.
| Materials and Methods | ▴Top |
This retrospective cohort study included patients 18 - 89 years old with MM who underwent ASCT at the Allegheny Health Network Cancer Institute between February 2016 and September 2021. Melphalan 200 mg/m2 (Mel200) or 140 mg/m2 (Mel140) was administered in divided doses on day -2 and -1 prior to cell infusion on day 0. The cohort was divided into two groups based on renal function, which was defined by CrCl (CrCl ≥ 60 mL/min (no-CKD) and CrCl < 60 mL/min (CKD)). CrCl was calculated using the Cockcroft-Gault formula [9]. Exclusion criteria included pregnant or incarcerated patients and patients with a diagnosis of amyloidosis. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [10]. The objective of this single institution study was to evaluate melphalan dosing and renal impairment on transplant-related outcomes, PFS, and OS. Neutrophil engraftment was defined as the first of three consecutive days of achieving a sustained peripheral blood neutrophil count of greater than 500 × 106 cells/L, and platelet engraftment was defined as independence from platelet transfusion for at least 7 days with a platelet count of more than 20 × 109 cells/L [11]. PFS was defined as time from ASCT to date of progression or death, whichever occurred first. OS was defined as time from ASCT to death. This study was approved by the Institutional Review Board at Allegheny Health Network Cancer Institute. Ethical compliance with human study was performed per the Institutional Review Board standard for a retrospective study.
Statistics
Summary statistics are presented as percentages for categorical data and median with interquartile range (IQR) for quantitative data. Univariate and multivariate logistic regression model was used to study predictors of grade 3 or 4 toxicities and represented as odds ratio (OR) and 95% confidence interval (CI). Survival estimates were performed using the Kaplan-Meier method, and survival differences were assessed using the log-rank test. The Cox regression model was used to adjust for baseline characteristics and assess predictors of PFS and OS and represented as hazard ratio (HR) and 95% CI. All statistical analyses were conducted with IBM SPSS Statistics Version 23.
| Results | ▴Top |
A total of 124 patients were included in this study, 108 no-CKD patients and 16 CKD patients. Median CrCl was 47 mL/min (7 - 59) in the CKD group, with one patient on hemodialysis, and median CrCl was 106 mL/min (62 - 208) in the no-CKD group (95% CI: 52.7 - 84.8; P ≤ 0.001). Baseline patient demographics are depicted in Table 1. Majority of patients were white males, median age 62 years (range 37 - 77), and stage 2 disease was categorized using the International Staging System (ISS), and standard risk cytogenetics. The median dose of CD34+ cells infused was 4.6 (2.1 - 8) × 106 cells/kg. Four patients had received prior ASCT, and most patients had at least a partial response to their disease at time of ASCT. In the CKD group, 12/16 (75%) patients had stage 3 CKD. Mel200 is our institutional standard for MM patients undergoing ASCT; however, dose reduction to 140 mg/m2 was permitted for renal dysfunction, performance status, patient comorbidities, or physician discretion. In the no-CKD group (CrCl ≥ 60 mL/min), 104/108 (96%) of patients received Mel200. In the remaining 4% of patients, doses were reduced to Mel140 due to physician discretion based on patient comorbidities and hypocellular bone marrow going into ASCT. In the CKD group (CrCl < 60 mL/min), 11/16 (69%) of patients received Mel200. The one patient on hemodialysis received reduced dose of Mel140.
 Click to view | Table 1. Baseline Characteristics |
For the CKD vs. no-CKD groups, the median time to neutrophil engraftment was 13.6 vs. 14.9 days (95% CI: 0.1 - 2.5; P = 0.03) and median time to platelet engraftment was 18.3 vs. 18.5 days (95% CI: 1.7 - 2.1; P = 0.8). Cox regression model was used to further adjust for melphalan dose reduction to 140 mg/m2, the median time to neutrophil or platelet engraftment was not statistically significant (P = 0.11 and 0.74, respectively).
At a median follow-up of 28.7 months, the median PFS for the CKD vs. no-CKD group was 60 vs. 46 months (P = 0.3) (Fig. 1). Median OS was not reached for both groups (P = 0.22) (Fig. 2). One-year OS was 93.8% vs. 97% in the CKD group vs. no-CKD group. When further adjusting for melphalan dose reduction to 140 mg/m2 and baseline characteristics (age, race, sex, and dose of CD34+ infusion), median PFS and OS were not statistically significant (P = 0.8 and 0.5, respectively). Increasing CD34+ stem cell dose was associated with lower risk of relapse (HR: 0.38; 95% CI: 0.21 - 0.68; P = 0.001). No predictors were found for OS in our study. One TRM event occurred in a CKD patient with a CrCl of 47 mL/min.
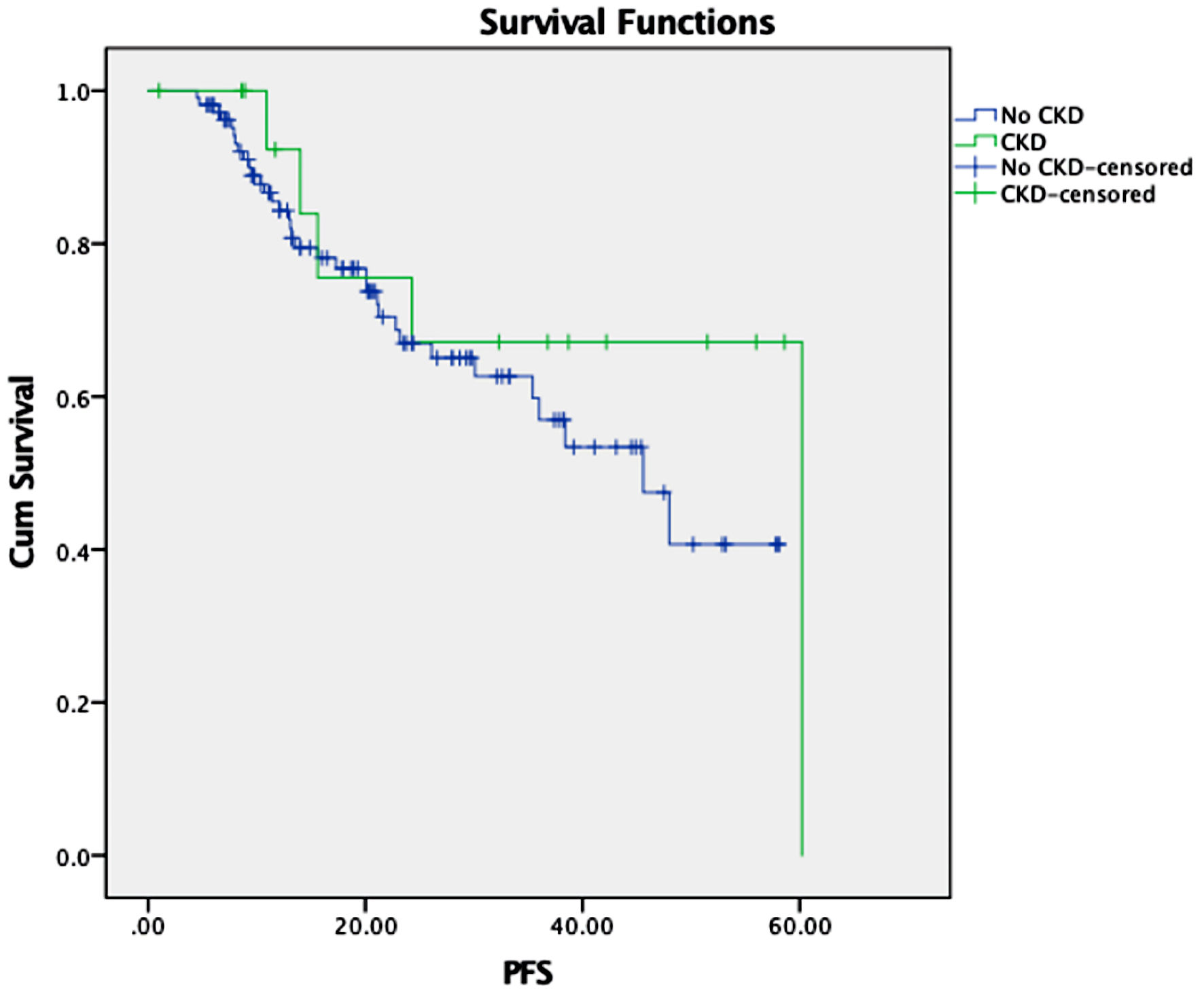 Click for large image | Figure 1. Kaplan’s survival function in months by renal function. CKD: chronic kidney disease; PFS: progression-free survival. |
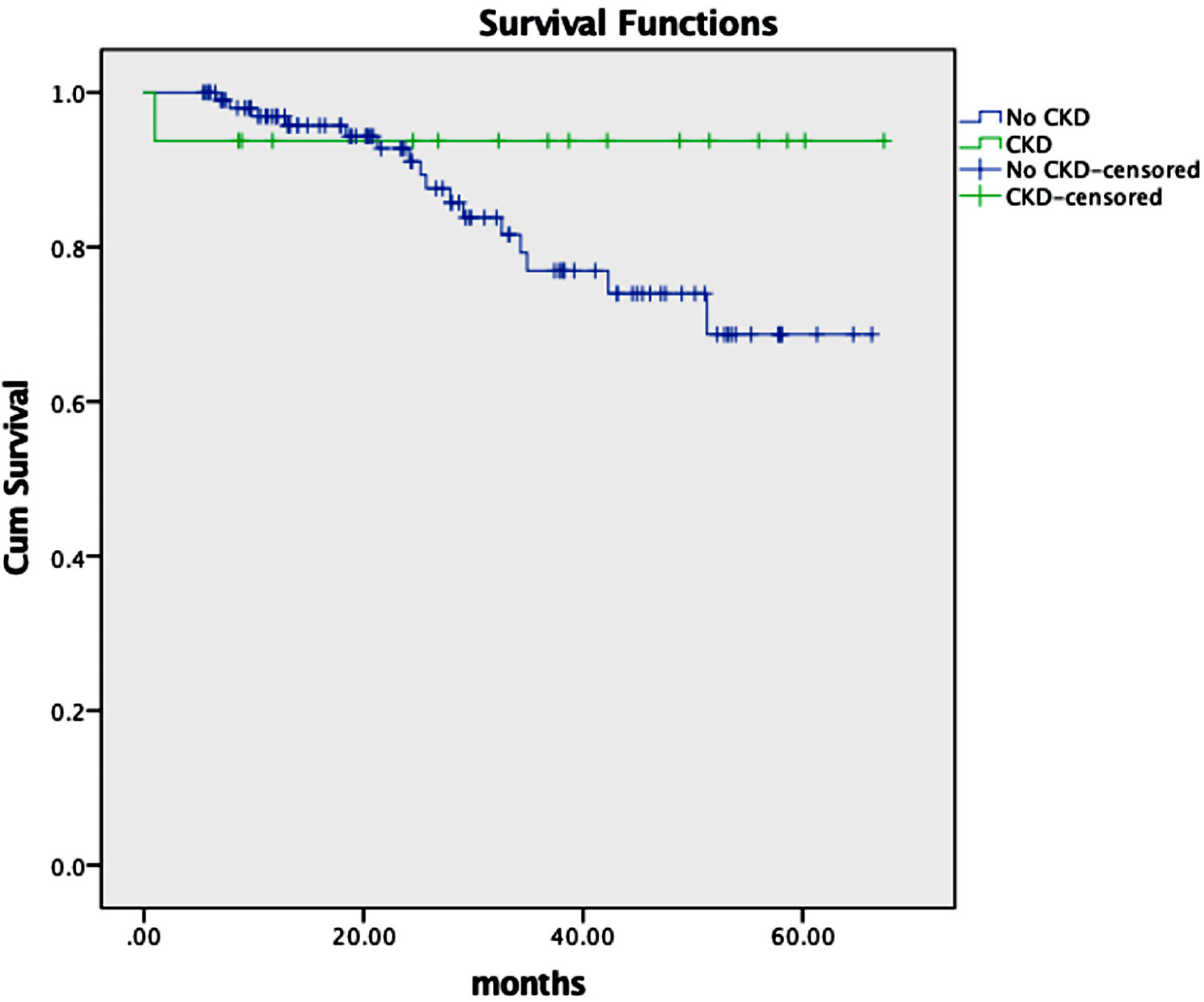 Click for large image | Figure 2. Kaplan’s overall survival function in months by renal function. CKD: chronic kidney disease. |
No significant differences in grade 3 or higher toxicity for diarrhea, nausea, or vomiting were observed between the CKD group vs. no-CKD group (P = 1, P = 0.6, P = 0.6, respectively). There was a higher incidence of grade 3 or 4 mucositis in the CKD group vs. no-CKD group (P = 0.01) (Fig. 3). Female sex (OR: 4.2; 95% CI: 1.1 - 16.4; P = 0.04) and having CKD (OR: 8.2; 95% CI: 1.4 - 47.2; P = 0.02) were associated with increased risk of developing grade 3 or 4 mucositis. Of note, melphalan dose was not a predictor of developing mucositis based on our statistical analysis using the Cox regression model. No statistically significant predictors were found for developing grade 3 or 4 nausea, vomiting, or diarrhea.
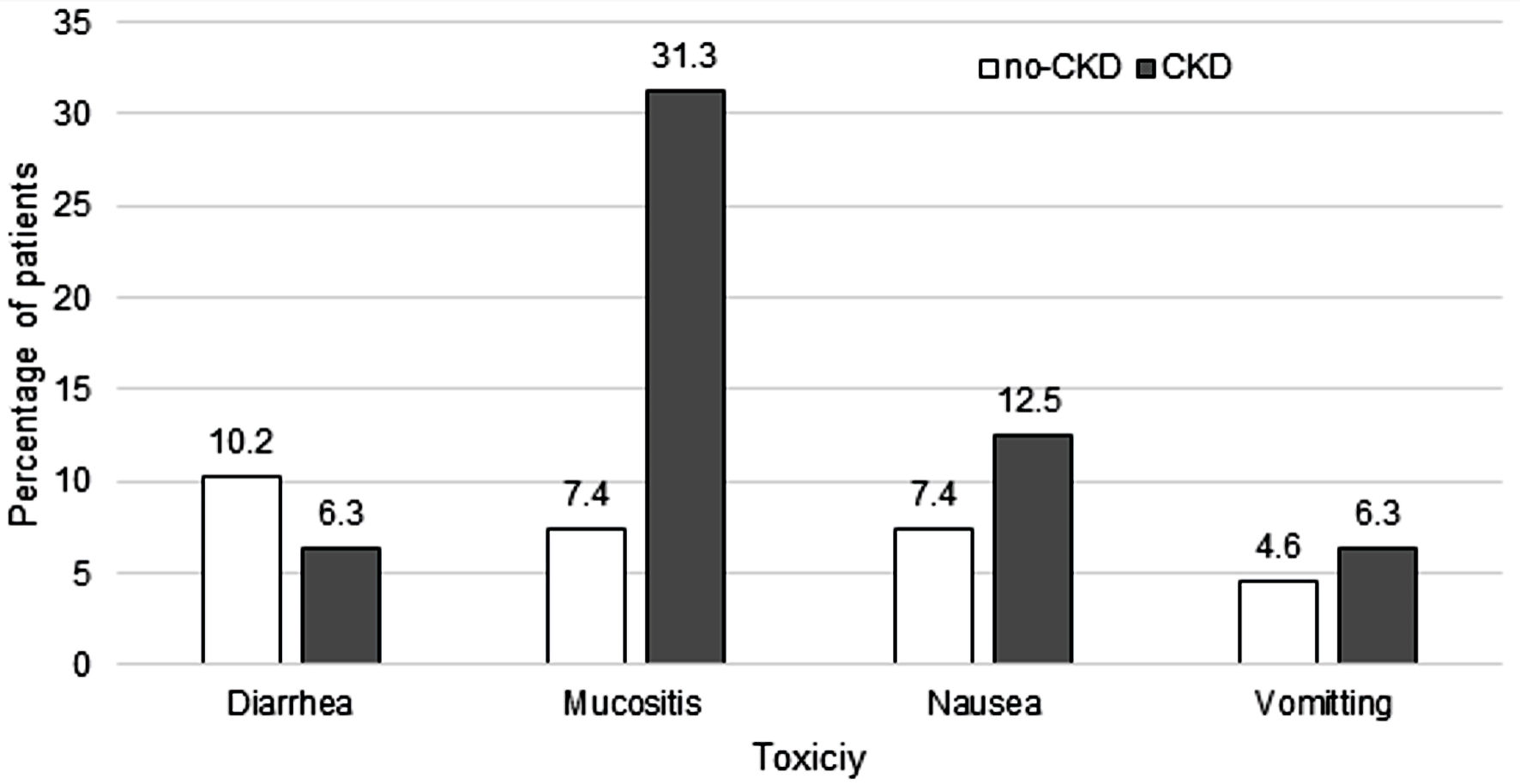 Click for large image | Figure 3. Grade 3 or 4 toxicities by renal function. CKD: chronic kidney disease. |
Other measures of toxicity included total parenteral nutrition (TPN) use, incidence of febrile neutropenia (FN), intravenous antibiotic use, and duration of hospitalization. No patients received TPN. FN occurred in 9/16 (56%) of patients in the CKD versus 53/108 (49%) in the no-CKD group (P = 0.79). Intravenous antibiotics were administered to 94% of FN patients. Hospital length of stay was similar between the CKD vs. no-CKD group (6 vs. 4 days, P = 0.35). Thirty-day readmissions to the hospital occurred in 9/16 (56%) of patients in the CKD versus 35/108 (32%) in the no-CKD group (P = 0.09). The most common reason for readmission was FN, followed by gastrointestinal adverse events, including mucositis.
| Discussion | ▴Top |
In this retrospective cohort study involving MM undergoing ASCT, there is no significant difference in engraftment, PFS, or OS between the CKD vs. no-CKD groups who received melphalan conditioning. Severe mucositis was significantly more common in the CKD group, as demonstrated by the incidence of grade 3 or 4 mucositis in the CKD group vs. no-CKD group (P = 0.01). Furthermore, female sex and having CKD were associated with increased risk of developing grade 3 or 4 mucositis. There were no other differences in toxicities between CKD and no-CKD group, even after adjusting for melphalan dose in our multivariate regression model. Our institutional standard is to use Mel200; however, physician discretion based on patient’s performance status, age and comorbidities was a reason for dose reducing to melphalan 140 mg/m2 (Mel140) in the setting of normal renal function. This occurred in four (4%) patients out of 108.
Autologous stem cell transplant remains standard of care in patients with newly diagnosed MM who are deemed transplant eligible. Mel200 is considered the standard conditioning dose; however, given concerns of potential toxicity, the reduced dose of Mel140 has been used in patients who are considered frail or have renal impairment. There are no randomized trials comparing these two regimens, and published literature has reported conflicting outcomes.
There are studies that support the use of Mel200 in elderly patients and renal impairment. Some studies did not show increased toxicity with melphalan dosing, however other published literature suggests the contrary [5, 6, 12, 13]. Results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study reported there were no significant differences between Mel200 versus Mel140 for OS, PFS, cumulative incidence of relapse, no-relapse mortality, and hematopoietic recovery. However, this study noted that disease state remission status at the time of transplant may have an impact with melphalan dosing for key transplant outcomes [6]. The CALM study also did not show any benefit of Mel200 over Mel140 in patients with high-risk cytogenetics or a higher ISS stage [6].
Regarding CKD in MM patients undergoing ASCT, only a few small retrospective studies have compared the effects of Mel200 vs. Mel140 in patients with renal impairment. A study in patients with renal failure showed no difference in renal recovery with Mel200 versus Mel140 and no impact on stem cell collections nor engraftment outcomes [14]. Another study showed mild improvement in event-free survival but no difference in complete remission or OS; moreover, in this particular study the dose of melphalan had to be reduced from 200 mg/m2 to 140 mg/m2 due to excess toxicities [15]. A study conducted by Sweiss et al showed that in patients with moderate renal impairment defined as CrCl 30 - 60 mL/min, Mel200 demonstrated improved treatment-free survival when compared to Mel140 (37 vs. 17 months) and similar OS, when compared to patients with normal CrCl, despite increased toxicity, delayed neutrophil, and platelet engraftment [5]. Srour et al in their large single-center study of 911 MM patients demonstrated that Mel140 had comparable efficacy to Mel200, and in the baseline characteristics of the Mel140 group, despite matching, had higher rates of renal insufficiency [16]. A retrospective cohort study reported that reduced doses of melphalan 140 or 100 mg/m2 had equivalent safety and efficacy for patients who are not candidates for Mel200. Renal impairment (65% of patients) was higher in the dose-reduced melphalan arm, but still contained some patients with normal renal function [17].
The response rates and OS after ASTCT with melphalan conditioning are known to be impacted by many factors including disease cytogenetics, ISS stage, and disease response at time of transplant. The CALM study results suggest that patients with poor response to induction chemotherapy obtain greater benefit from Mel200 over Mel140; however, patients who have achieved very good partial response (VGPR) or complete response (CR) to induction regimen do better with Mel140 compared to Mel200. Given the retrospective nature of our study, this finding could not be fully explained [6]. One possible explanation could be melphalan pharmacokinetics. CrCl, fat free mass and hematocrit are some of the factors that have been shown to affect melphalan pharmacokinetics and may in turn affect toxicity and responses [18]. High melphalan exposure has been linked to improved OS in patients undergoing ASCT, but not to time to progression or PFS. These findings suggest that melphalan exposure alone is not a direct measure of its anti-myeloma effect [18, 19].
It is important to note that some of these studies were conducted before the availability of novel agents, which have considerably improved response rates in patients prior to ASCT. With improved response rates prior to ASCT, providers may feel more comfortable reducing melphalan doses compared to historical trends. As melphalan is partially renally excreted, CrCl will influence toxicity, especially in patients with other medical comorbidities [13]. Given the potential toxicity, it is not uncommon for transplant centers to use lower doses of melphalan in clinical practice for patients with renal impairment and multiple comorbid conditions.
In our study, when comparing disease status prior to undergoing ASCT, six (40%) patients in the CKD group had achieved CR or VGPR, and 43 (38%) patients achieved CR or VGPR in the no-CKD group. When compared to melphalan dosing, more patients (5/7) in the CKD cohort received Mel140 and underwent ASCT in partial response or stable disease status as compared to only two patients in the no-CKD cohort, who received Mel140 and were in CR or VGPR. Since the responses to induction regimen were almost similar in both the groups, we believe that based on our findings, the depth of response when adjusted to melphalan dosing did not alter the outcomes following ASCT. This finding also suggests that the choice of induction regimen does impact the initial response but may have little impact on post-transplant outcomes irrespective of the melphalan dosing. Limitations of our study include small sample size and its retrospective design. The CKD group had 16 patients and only one patient was on intermittent hemodialysis prior to ASCT, so it would be difficult to apply our results to patients with CKD and on hemodialysis.
In conclusion, Mel140 can be considered a reasonable conditioning regimen in patients with MM who are considered at risk of increased toxicity. Remission status at the time of first ASCT should also be considered when determining the melphalan dose. A randomized control study is needed to compare Mel200 to Mel140 in renal impairment to achieve broader applicability.
Acknowledgments
There were no specific acknowledgments to be mentioned.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
Salman Fazal: Consultant for BMS, PharmaEssentia, Taiho, Blueprint, Pfizer, Incyte, Janssen, GSK and Speaker for BMS, PharmaEssentia, Taiho, Blueprint, Pfizer, Jazz, Incyte, Janssen, GSK, Takeda, Stemline, Omeros, Servier, Takeda, Gilead, and Novartis.
Informed Consent
Not applicable.
Author Contributions
Sorana G. Ursu contributed to research question idea creation, data collection, analysis of data collected, wrote abstract, methods and results section of the manuscript, prepared manuscript for publication per journal submission requirement, incorporated edits from co-authors into final manuscript draft, created Table 1 and Figure 3, and wrote the cover letter. Samantha Maples contributed to data collection, wrote background section of the manuscript, edited entire manuscript. Kiersten J. Williams contributed to data collection and edited the entire manuscript. Gina Patrus contributed to data collection and edited the results section of the manuscript. Yazan Samhouri contributed to statistical analysis performed using software, created Figures 1 and 2, and edited the entire manuscript. Salman Fazal and Prerna Mewawalla edited the entire manuscript. Santhosh Sadashiv wrote the discussion section of the manuscript, contributed to analysis of data collected, and edited the entire manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ASCT: autologous stem cell transplantation; CKD: chronic kidney disease; CALM: Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma; CTCAE: Common Terminology Criteria for Adverse Events; CR: complete response; CI: confidence interval; CrCl: creatinine clearance; FN: febrile neutropenia; ISS: International Staging System; IQR: interquartile range; Mel200: melphalan 200 mg/m2; Mel140: melphalan140 mg/m2; MM: multiple myeloma; OS: overall survival; PFS: progression-free survival; TPN: total parenteral nutrition; TRM: treatment-related mortality; VGPR: very good partial response
| References | ▴Top |
- Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(3):344-356.
doi pubmed pmc - Bodge MN, Reddy S, Thompson MS, Savani BN. Preparative regimen dosing for hematopoietic stem cell transplantation in patients with chronic kidney disease: analysis of the literature and recommendations. Biol Blood Marrow Transplant. 2014;20(7):908-919.
doi pubmed - Alkeran® [package insert]. Research Triangle Park, NC: GlaxoSmithKlin; 2011.
- Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, Vesole DH, et al. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996;2(6):947-952.
pubmed - Sweiss K, Patel S, Culos K, Oh A, Rondelli D, Patel P. Melphalan 200 mg/m(2) in patients with renal impairment is associated with increased short-term toxicity but improved response and longer treatment-free survival. Bone Marrow Transplant. 2016;51(10):1337-1341.
doi pubmed - Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH, Apperley JF, et al. Melphalan 140 mg/m(2) or 200 mg/m(2) for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica. 2018;103(3):514-521.
doi pubmed pmc - Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, McLachlan AJ, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol. 2010;69(5):484-497.
doi pubmed pmc - Sweiss K, Calip GS, Johnson JJ, Rondelli D, Patel PR. Pretransplant hemoglobin and creatinine clearance correlate with treatment-free survival after autologous stem cell transplantation for multiple myeloma. Bone Marrow Transplant. 2019;54(12):2081-2087.
doi pubmed pmc - Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
doi pubmed - National Cancer Institute. Common terminology criteria for adverse events v5.0 NCI, NIH, DHHS. Bethesda: NIH Publication. 2017.
- Hutt D. Engraftment, Graft Failure, and Rejection. In: Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses: under the auspices of EBMT. Cham (CH), 2018; 259-270.
doi pubmed - Garderet L, Beohou E, Caillot D, Stoppa AM, Touzeau C, Chretien ML, Karlin L, et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica. 2016;101(11):1390-1397.
doi pubmed pmc - Katragadda L, McCullough LM, Dai Y, Hsu J, Byrne M, Hiemenz J, May S, et al. Effect of melphalan 140 mg/m(2) vs 200 mg/m(2) on toxicities and outcomes in multiple myeloma patients undergoing single autologous stem cell transplantation-a single center experience. Clin Transplant. 2016;30(8):894-900.
doi pubmed - Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R, Talamo G, et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 2004;33(8):823-828.
doi pubmed - Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, Desikan R, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114(4):822-829.
doi pubmed - Srour SA, Milton DR, Bashir Q, Nieto Y, Saini N, Daher M, Ramdial J, et al. Melphalan dose intensity for autologous stem cell transplantation in multiple myeloma. Haematologica. 2021;106(12):3211-3214.
doi pubmed pmc - Ngo PT, Reynolds SB, Nguyen QL, Jayswal R, Weiss HL, Krem MM. Reduced-dose melphalan (140 or 100 mg/m2) maintains efficacy and tolerability for multiple myeloma patients with advanced age or renal impairment undergoing auto-hct. Biology of Blood and Marrow Transplantation. 2019;25(3):S24-S25.
- Shaw PJ, Nath CE, Lazarus HM. Not too little, not too much-just right! (Better ways to give high dose melphalan). Bone Marrow Transplant. 2014;49(12):1457-1465.
doi pubmed - Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I, Kwan YL, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol. 2016;82(1):149-159.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


