| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 13, Number 1-2, April 2024, pages 12-22
Effective Management of Polycythemia Vera With Ropeginterferon Alfa-2b Treatment
Shan Shan Suoa, s, Rong Feng Fub, s, Albert Qinc, t, Zong Hong Shaod, Jie Baid, Su Ning Chene, Ming Hui Duanf, Hu Zhoug, Na Xuh, Su Jiang Zhangi, Xue Lan Zuoj, Xin Duk, Li Wangl, Pei Lim, Xu Han Zhangn, Dao Xiang Wuo, Ya Ning Lio, Jing Jing Zhango, Wei Wango, Wei Hong Sheno, Oleh Zagrijtschukp, Toshiaki Satoq, Zhi Jian Xiaor, Jie Jina, t
aThe First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
bState Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China
cMedical Research & Clinical Operations, PharmaEssentia Corporation, Taipei, Taiwan, Republic of China
dThe Second Hospital of Tianjin Medical University, Tianjin, China
eThe First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
fPeking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
gAffiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan, China
hNanfang Hospital of Southern Medical University, Guangzhou, Guangdong, China
iRuijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
jZhongnan Hospital, Wuhan University, Wuhan, Hubei, China
kShenzhen Second People’s Hospital, Shenzhen, Guangdong, China
lThe First Affiliated Hospital of Chongqing Medical University, Chongqing, China
mHuashan Hospital of Fudan University, Shanghai, China
nThe First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
oPharmaEssentia Biotech (Beijing) Limited, Beijing, China
pPharmaEssentia USA Corporation, Burlington, MA, USA
qPharmaEssentia Japan K.K., Motoakasaka, Minato-ku, Tokyo, Japan
rInstitute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China
sThese authors contributed equally to this study.
tCorresponding Author: Jie Jin, Department of Hematology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, Chinaand Albert Qin, Medical Research & Clinical Operations, PharmaEssentia Corporation, Taipei 115, Taiwan, Republic of China
Manuscript submitted January 24, 2024, accepted March 7, 2024, published online April 9, 2024
Short title: Ropeginterferon Alfa-2b Treatment for PV
doi: https://doi.org/10.14740/jh1245
| Abstract | ▴Top |
Background: Polycythemia vera (PV) is a myeloproliferative neoplasm. Ropeginterferon alfa-2b is a new-generation polyethylene glycol-conjugated proline-interferon. It is approved for the treatment of PV at a starting dose of 100 µg (50 µg for patients receiving hydroxyurea (HU)) and dose titrations up to 500 µg by 50 µg increments. The study was aimed at assessing its efficacy and safety at a higher starting dose and simpler intra-patient dose escalation.
Methods: Forty-nine patients with PV having HU intolerance from major hospitals in China were treated biweekly with an initial dose of 250 µg, followed by 350 µg and 500 µg thereafter if tolerated. Complete hematological response (CHR) was assessed every 12 weeks based on the European LeukemiaNet criteria. The primary endpoint was the CHR rate at week 24. The secondary endpoints included CHR rates at weeks 12, 36 and 52, changes of JAK2V617F allelic burden, time to first CHR, and safety assessments.
Results: The CHR rates were 61.2%, 69.4% and 71.4% at weeks 24, 36, and 52, respectively. Mean allele burden of the driver mutation JAK2V617F declined from 58.5% at baseline to 30.1% at 52 weeks. Both CHR and JAK2V617F allele burden reduction showed consistent increases over the 52 weeks of the treatment. Twenty-nine patients (63.0%) achieved partial molecular response (PMR) and two achieved complete molecular response (CMR). The time to CHR was rapid and median time was 5.6 months according to central lab results. The CHRs were durable and median CHR duration time was not reached at week 52. Mean spleen index reduced from 55.6 cm2 at baseline to 50.2 cm2 at week 52. Adverse events (AEs) were mostly mild or moderate. Most common AEs were reversible alanine aminotransferase and aspartate aminotransferase increases, which were not associated with significant elevations in bilirubin levels or jaundice. There were no grade 4 or 5 AEs. Grade 3 AEs were reversible and manageable. Only one AE led to discontinuation. No incidence of thromboembolic events was observed.
Conclusion: The 250-350-500 µg dosing regimen was well tolerated and effectively induced CHR and MR and managed spleen size increase. Our findings demonstrate that ropeginterferon alfa-2b at this dosing regimen can provide an effective management of PV and support using this dosing regimen as a treatment option.
Keywords: Ropeginterferon alfa-2b; Polycythemia vera; Complete hematological response; Molecular response; JAK2V617F allele burden; Hydroxyurea resistance or intolerance
| Introduction | ▴Top |
Polycythemia vera (PV) is a burdensome, myeloproliferative neoplasm (MPN). Most patients suffering from PV carry the driver Janus kinase 2 gene (JAK) mutation JAK2V617F. PV is associated with an over-production of blood cells, increased incidence of thromboembolic and hemorrhagic complications, and long-term risk of transformation to myelofibrosis (MF) or acute myeloid leukemia (AML) [1-3]. Patients suffering from PV have lower rates of survival and the incidence rate of thrombotic, fibrotic, or leukemic events, which are the main causes of morbidity and mortality, at 20 years is 26%, 16%, and 4%, respectively [4, 5]. Age and history of thrombotic events (TEs) were the factors included in the conventional risk model to determine the risk of thrombosis. In addition, elevated hematocrit (HCT) levels have been demonstrated to have a close relationship with TEs. Several recent studies have also shown the relevance of increased TE risk to white blood cell (WBC) and platelet (PLT) counts, and JAK2 variant allelic frequency (VAF) [6, 7]. Therefore, the clinical management of PV includes maintaining HCT levels < 45%, achieving complete hematological response (CHR), and reducing the driver JAK2 mutation VAF to reduce the risk of TEs, and mitigating disease progression to MF and AML.
Ropeginterferon alfa-2b (BESREMi®) is a novel, site-selective polyethylene glycol-conjugated (PEGylated) recombinant proline-interferon-alpha with a favorable pharmacokinetic (PK) profile [8-11]. Its clinical efficacy and safety in the treatment of PV, regardless of race or ethnic background, has been demonstrated in several clinical studies [12-18]. It has been approved for the treatment of adult PV patients in the USA, Europe, and other countries or regions [19, 20]. It is the only interferon (IFN)-based therapy that has been approved by the US Food and Drug Administration (FDA) for treating PV and can be used regardless of the risk category or previous treatment history [19]. Ropeginterferon alfa-2b is used biweekly with an initiating dose of 100 µg (or 50 µg for patients receiving hydroxyurea (HU)), which is followed by 50 µg increments up to the maximum dose of 500 µg. The slow-titration schema with a low starting dose followed by 50 µg intra-patient dose increments could take up to approximately 6 months or more to attain the plateau dose level. During this period of slow dose titration, the hematological parameters and JAK2V617F might not be optimally controlled, and patients might potentially face a thrombotic or hemorrhagic risk resulting from inadequate drug exposure [21]. We designed a phase II study to primarily evaluate whether initiating ropeginterferon alfa-2b at a higher dose with a simpler and faster intra-patient dose titration, i.e., the 250-350-500 µg dosing regimen, could result in an efficient CHR rate and molecular response (MR) in PV patients at 6 months based on the existing safety data in other indications for ropeginterferon alfa-2b, including viral hepatitis [21-25]. The results demonstrated that a CHR rate of 61.7% was achieved by this dose regimen in 6 months, which was notably higher than previously reported with the slow-titration schema [26]. However, the longer-term efficacy including CHR and MR, safety, and patient discontinuation rate needed to be investigated. We hereby present the 1-year data for ropeginterferon alfa-2b treatment at the 250-350-500 µg dosing regimen, which indicates the feasibility and effectiveness of this regimen as a treatment option for patients with PV.
| Materials and Methods | ▴Top |
The phase II, multicenter, single-arm study was conducted at 15 major hospitals in China. Jin et al have previously described the details of the methods and clinical trial information [25]. The phase II study planned to enroll 49 patients with PV who were resistant or intolerant to HU treatment according to the European LeukemiaNet (ELN) criteria [27]. The patients were subcutaneously administered with ropeginterferon alfa-2b once every two weeks. The treatment protocol included a starting dose of 250 µg at week 0, followed by an intra-patient dose escalation to 350 µg at week 2, and then a dose of 500 µg at week 4 thereafter. Dose could be adjusted according to tolerability. The maintenance dose was 500 µg for up to 52 weeks if tolerated. The primary endpoint was CHR rate at week 24. CHR is defined as: HCT < 45% without phlebotomy or erythrocyte apheresis in the preceding 3 months, PLT count ≤ 400 × 109/L, and leukocyte count < 10 × 109/L. The secondary endpoints included CHR rates at weeks 12, 36 and 52; changes of JAK2V617F allelic burden from baseline; time to first CHR; and safety assessments including the incidence, causality and severity of adverse events (AEs) assessed according to the Common Terminology Criteria for Adverse Events 5.0 (CTCAE 5.0); the incidence, causality, and severity of AEs of special interest; and the events leading to dose reduction or permanent termination of treatment. Each patient was planned to be in the study for approximately 14 months, encompassing a 28-day screening phase, a 52-week treatment phase, and a 28-day safety follow-up period. Quantitative determination of JAK2V617F allele burden and hematological parameters for CHR assessment were carried out by a central laboratory. The 95% confidence interval (CI) of the CHR rate was estimated using the Clopper-Pearson method. The median and 95% CI of the time-event endpoints were estimated using the Kaplan-Meier method. The trial is registered at ClinicalTrials.gov (identifier: NCT05485948) and in China (China National Medical Products Administration registration number: CTR20211664). The institutional review board or ethics committee of all participating clinical sites have approved this study. Written informed consent was obtained from all participating patients. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient demographics, baseline characteristics and disposition
Forty-nine patients with PV were enrolled in this study. The mean age at enrollment was 53.0 years. All patients had previously received and had become intolerant to HU treatment according to the ELN criteria [27]. Of the patients, 61.2% had previously received an IFN-based therapy [26]. All patients needed cytoreductive therapy at baseline. Approximately 30.6% of the patients had previously suffered either a thrombosis or a hemorrhage. The driver mutation JAK2V617F was present in all patients with a mean baseline allelic burden of 58.5% (Table 1).
 Click to view | Table 1. Patient Demographics and Baseline Characteristics |
Forty-nine patients were enrolled in the intent-to-treat (ITT) and safety populations, among which two patients withdrew from the study due to personal reasons, one before completing the 24-week treatment and one after completing the 24-week treatment. AEs led to discontinuation of one patient and 46 patients completed the 52-week treatment regimen according to the protocol.
CHR rates, durability, and time to CHR
The CHR rates, based on the central laboratory assessments, at 12, 24, 36, and 52 weeks of the treatment were 44.9% [26], 61.2% [26], 69.4%, and 71.4%, respectively. Previously, a CHR rate of 43% following 1 year of treatment with ropeginterferon alfa-2b at the slow-titration schema was reported in the PROUD-PV study [16]. The CHR rates in the ITT population at different assessment visits are depicted in Figure 1a.
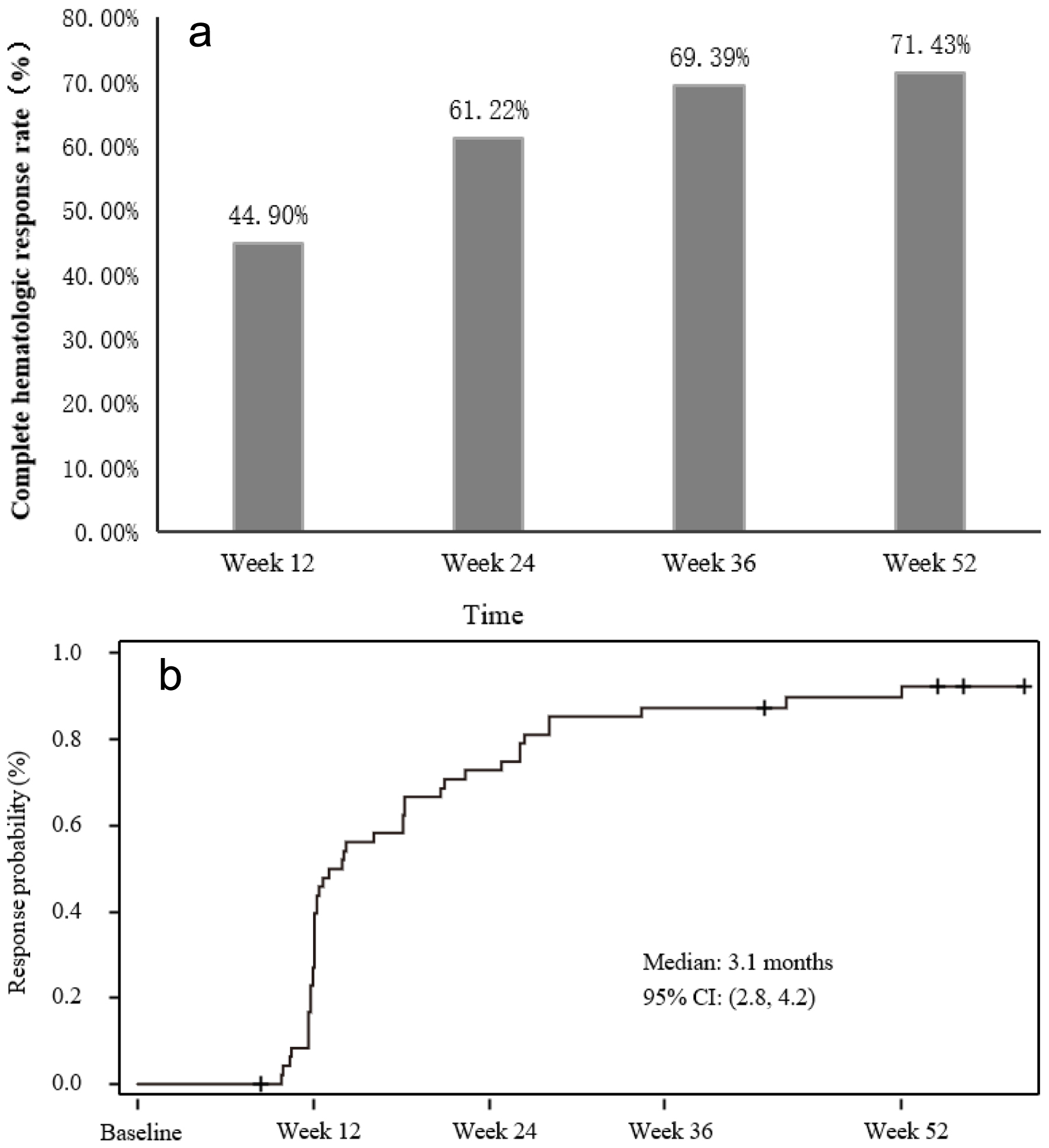 Click for large image | Figure 1. Complete hematological response (CHR) rates and time to CHR. (a) CHR rates at different assessment visits. (b) Graph depicting time to CHR. |
The durable CHR in this study was observed in 17 (34.7%) and 20 patients (40.8%), from week 24 to 36, and week 36 to 52, respectively. Durable CHR is defined as CHR lasting for at least 12 weeks according to the central lab measurements. Furthermore, patients need to have the CHR for all the local lab results available within the 12 weeks. The rates of the durable CHR increased over time and were numerically higher than previously observed in the PV studies with ropeginterferon alfa-2b at the slow-titration schema. For example, the rate of durable CHR from week 36 to 52 was observed to be 27.6% in a phase II study with ropeginterferon alfa-2b at the slow-titration schema [18].
Mean HCT, PLT, and WBC levels decreased over time from baseline to week 52 in this study: -5.3%, -282.8 × 109/L, and -6.5 × 109/L, respectively. After 52 weeks of treatment with ropeginterferon alfa-2b, all mean levels of the three peripheral hematological parameters improved to the normal ranges.
The overall CHR of the study was 83.7% with 41 patients achieving a CHR during 52 weeks of the treatment, according to central lab results, which were measured on the assessment visits (once every 12 weeks). The median time to achievement of the first CHR was 5.6 months (95% CI: 2.9 - 5.7) according to the central lab assessments. Based on the local lab measurements which were more frequent, the median time to CHR was 3.1 months (95% CI: 2.8 - 4.2) (Fig. 1b). Previously, the time to CHR with ropeginterferon alfa-2b at the slow-titration schema was observed to be approximately 12 months [18].
Overall CHR duration was further assessed as the number of days between the initiation of CHR and the criteria for CHR being no longer met according to local lab measurements. Forty-four patients (89.8%) achieved at least one CHR during the 52 weeks of the treatment according to local lab assessments. CHR was maintained in 29 patients (59.2%) from the first CHR until the end of the 52-week treatment. As demonstrated in Figure 2, the CHR was very durable, which is evident from the fact that median duration of CHR for patients with at least one response at any visit during the study was not reached at week 52. These results indicate that ropeginterferon alfa-2b at the 250-350-500 µg dosing regimen, which has a higher initiating dose and simpler dose titration, can lead to high levels of CHR, which also occur rapidly and are very durable.
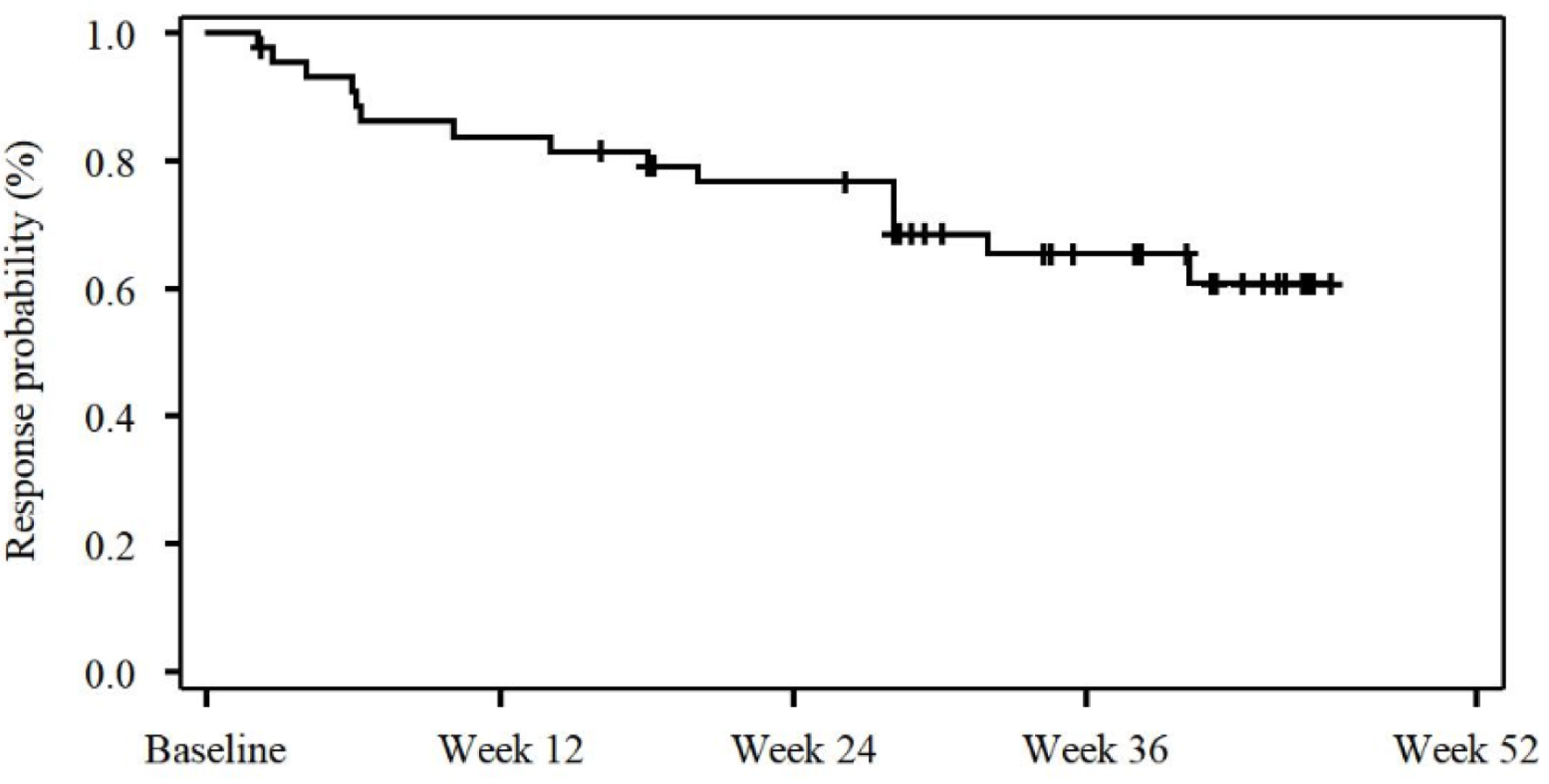 Click for large image | Figure 2. Kaplan-Meier plot demonstrating the complete hematological response (CHR) duration. |
Spleen index
Spleen index was defined as the maximum length on the longitudinal sonogram (cm) times thickness on the transverse sonogram (cm) by ultrasound. The change of spleen index (cm2) was assessed at week 52 and compared with the baseline measurement. Forty-two patients with measurable baseline values were evaluated for spleen response. After the 52-week ropeginterferon alfa-2b treatment, the mean spleen index reduced to 50.2 cm2 with a mean spleen size reduction of 5.6 cm2, as shown in Figure 3. This result indicates that ropeginterferon alfa-2b at the 250-350-500 µg dosing regimen can control the spleen size increase in patients with PV.
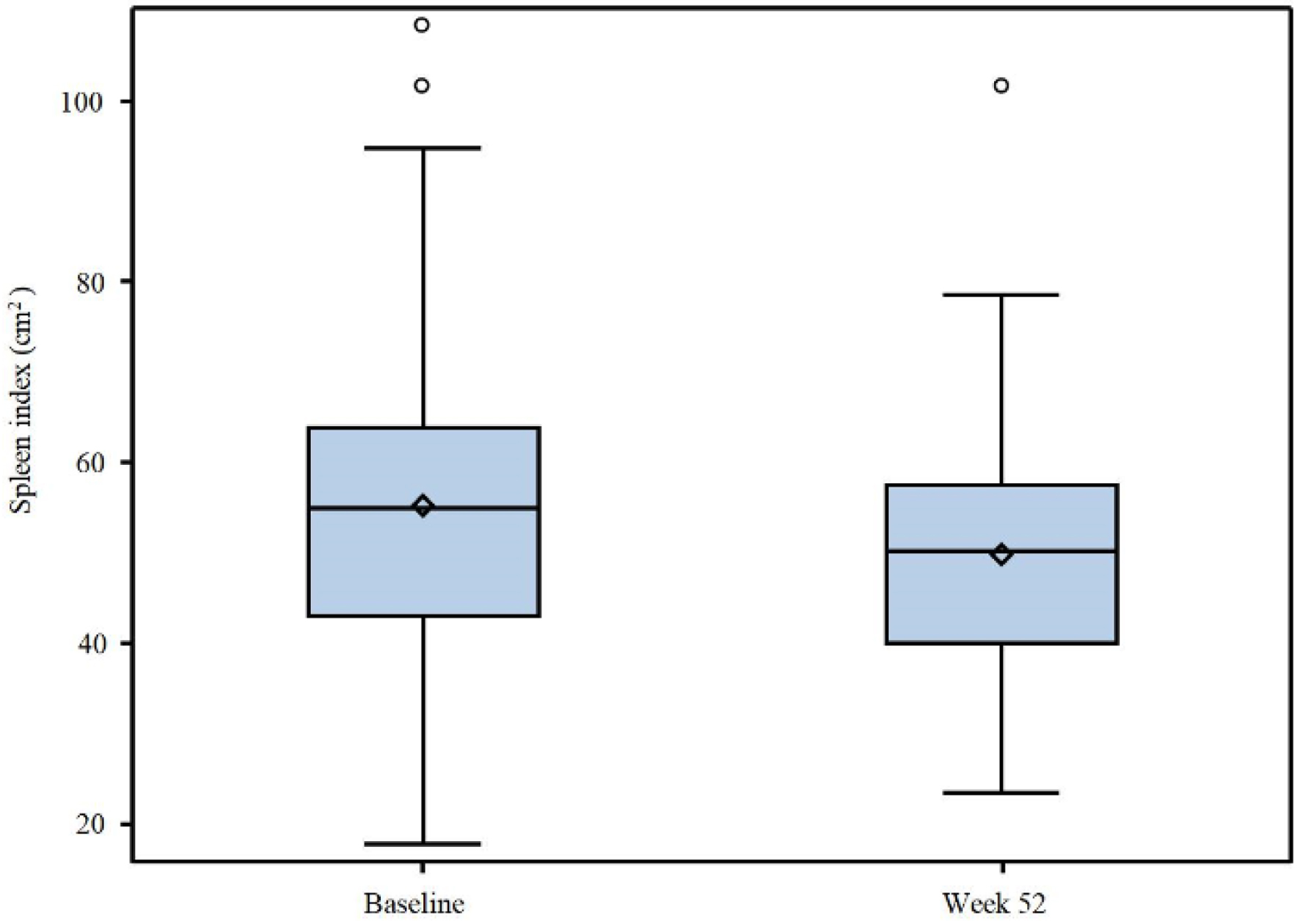 Click for large image | Figure 3. Graph demonstrating the mean spleen index change. The diamond indicates the mean value. |
JAK2V617F MR
After the 52-week treatment with ropeginterferon alfa-2b, the mean JAK2V617F allele burden decreased to 30.1% from 58.5% at the baseline. The median JAK2V617F allelic burden decreased from 61.2% at baseline to 18.8% at week 52 (Fig. 4a). The waterfall plot (Fig. 4b) indicates a reduction in JAK2V617F allelic burden in 42/46 patients (91.3%) during the 52 weeks of the treatment. Two patients demonstrated CMR at week 52 as measured by next-generation sequencing technology [28] with a lower limit of quantitation at 1% by the central laboratory. One patient had a baseline JAK2V617F allelic burden of 59.2% and the other had 4.7%. The JAK2V617F allelic burden was undetectable for both patients after 52 weeks of treatment. Based on the 2009 criteria [29], 29 patients (63.0%) demonstrated PMR, defined as a reduction ≥ 50% in patients with < 50% mutant allele burden, or a reduction ≥ 25% in patients with > 50% mutant allele burden. According to the 2013 ELN criteria [30], 18 patients (39.1%) achieved PMR, defined as ≥ 50% decrease in allele burden in patients with at least 20% mutant allele burden at baseline. The JAK2V617F allelic burden of 13 patients (28.3%) decreased to less than 10% following 52 weeks of treatment.
 Click for large image | Figure 4. The effect of ropeginterferon alfa-2b on JAK2V617F allelic burden. (a) Graph demonstrating the change of median JAK2V617F allelic burden (%) during the 52 weeks of treatment. (b) Waterfall plot indicating the change of the JAK2V617F allelic burden in individual patients after 52 weeks of treatment. Blue color represents the patients who had complete hematological response (CHR). |
Safety
All 49 patients were included in the safety analysis. All except one patient reached the dose of 500 µg over the 52 weeks of treatment. The median duration of exposure to ropeginterferon alfa-2b was 365 days. Including the first 4 weeks of intra-patient dose escalations from 250 to 500 µg, the mean dose per patient over 52 weeks was 435.2 ± 59.2 µg once every 2 weeks. The median dose per patient was 462.8 µg once every 2 weeks over the 52 weeks.
Most treatment-emergent AEs were mild or moderate (Table 2). Elevated alanine aminotransferase (ALT) levels (28/49, 57.1%) and aspartate aminotransferase (AST) increase (28/49, 57.1%), WBC count decrease (23/49, 46.9%), hyperuricemia (22/49, 44.9%), gamma-glutamyl transferase (GGT) increase (20/49, 40.8%), neutrophil count decrease (18/49, 36.7%), and hypertriglyceridemia (18/49, 36.7%) were the most common AEs encountered. Most of the AEs were grade 1 or 2 and were reversible. No grades 4 or 5 AEs were observed in the study. Although ALT and AST elevations are common, only one patient had a grade 3 ALT elevation, and the patient did not have elevated bilirubin levels or jaundice. Possible drug-related grade 3 AEs included GGT increase (2/49, 4.1%), lymphocyte count decrease (2/49, 4.1%), neutrophil count decrease (2/49, 4.1%), WBC count decrease (1/49, 2.0%), ALT increase (1/49, 2.0%), pneumonia (1/49, 2.0%), arthralgia and peripheral edema (1/49, 2.0%), and hypertriglyceridemia (1/49, 2.0%). All grade 3 AEs except for one patient who had a grade 3 lymphocyte count decrease, recovered or were resolved. The grade 3 AE of lymphocyte count decrease improved to the grade 2 level. Serious AEs (SAEs) including pneumonia, ALT and AST increase, and myasthenia gravis that were possibly related were observed in three patients. Only one patient discontinued the study due to an AE (grade 2 myasthenia gravis). Five patients showed AEs of special interest (10.2%), including two cases of grade 1 and 2 conjunctival hyperemia (4.1%), respectively, one case of grade 2 diarrhea (2%), one grade 2 hyperthyroidism (2%), and one grade 2 myasthenia gravis (2%). No patient experienced a thromboembolic event and there was no occurrence of transformation to MF or AML.
 Click to view | Table 2. Treatment-Emergent Adverse Events Occurring in ≥ 10% Patients |
PK analysis
PK parameters were estimated following administration of the starting dose of 250 µg and intra-patient dose escalation to 500 µg based on the non-compartmental model. Descriptive analyses of concentrations and PK parameters were performed. The summary of PK parameters is given in Table 3. The PK parameters of ropeginterferon alfa-2b following the single dose of 250 µg in this study were observed to be similar to those of a single dose of 270 µg in healthy volunteers [31]. The median time to the maximum serum concentration (Tmax) in this study and health volunteers was 96.0 vs. 132.0 h. The area under the serum concentration-time curve from time 0 to 336 h (AUC0-336 h) was 4,082,160 vs. 4,838,047 h × pg/mL and maximum serum concentration (Cmax) was 18,632 vs. 24,140 pg/mL. The Cmax and AUC after intra-patient dose titrations (500 µg) in this study were higher than those after the initial dose of 250 µg (Table 3), which is consistent with the intra-patient dose escalation.
 Click to view | Table 3. Summary of PK Parameters |
| Discussion | ▴Top |
The results in our study demonstrate that the new dosing regimen of 250-350-500 µg of ropeginterferon alfa-2b is well-tolerated and highly efficacious in patients suffering from PV. This dosing regimen was associated with a rapid achievement of CHR without the need for phlebotomy or erythrocyte apheresis. The CHRs were very durable and the levels of CHR were numerically greater than previously reported for the slow-titration dosing schema. This was also accompanied with a high level of JAK2V617F allelic burden reduction. The results suggested that treatment of patients with PV at this dosing regimen of a higher starting dose and simpler intra-patient dose titration can eradicate the mutant cell clones carrying the driver mutation JAK2V617F in PV in a more efficient manner. Both hematological parameters including HCT, WBC and PLT counts, as well as JAK2V617F are potentially risk factors for patients to develop TEs [32-37]. JAK2V617F can also contribute to the neoplastic progression to MF and AML [36-38]. In addition, ropeginterferon alfa-2b treatment appeared to control the spleen size increase with the mean spleen index decreasing from 55.6 cm2 at baseline to 50.2 cm2 following 52 weeks of the treatment in this study. Inhibition of disease progression and prolonged progression- or event-free survival in patients with PV could be attributed to PEGylated IFN-based therapies [39, 40]. Thus, the ropeginterferon alfa-2b treatment at the 250-350-500 µg dosing regimen could potentially serve as an effective treatment option for patients with PV.
It is conceivable that at this dosing regimen, ropeginterferon alfa-2b is effective in all PV patients despite the fact that this study enrolled HU-intolerant patients. The Myeloproliferative Disorders Research Consortium studies MPD-RC 111 and 112 with PEGylated IFN alfa-2a indicated that the rate of CHR in PV patients with HU-resistance or -intolerance was comparable to that observed in treatment-naive patients at 12 months (22% and 28%, respectively) [41, 42]. Ropeginterferon alfa-2b has been approved for adult PV patients regardless of prior treatment history or disease risk category. It has demonstrated clinical efficacy in HU-naive as well as -previously treated patients in the phase III PROUD-PV/CONTINUATION-PV studies [16]. Furthermore, ropeginterferon alfa-2b works differently from HU, since it is an IFN-based therapy. The efficacy of ropeginterferon alfa-2b could potentially be attributed to the selective inhibition of the driver mutation JAK2V617F-carrying malignant hemopoietic stem or progenitor cells [43], while HU can be non-specific and induce DNA damage. In this study, ropeginterferon alfa-2b treatment at the 250-350-500 µg dosing schema showed a rapid time to achieve the CHR and the levels of CHR were high over the 52 weeks of treatment. Therefore, ropeginterferon alfa-2b at this dosing schema represents an effective treatment regimen for all PV patients, regardless of HU-resistance or intolerance.
In this study, a decrease in JAK2V617F allelic burden was observed in almost all patients (91.3%) during the 52 weeks of treatment. The mean reduction from 58.5% at baseline to 30.1% at 52 weeks was observed. Previously, a reduction from 41.9% at baseline to 30.7% at 52 weeks was observed with ropeginterferon alfa-2b at the slow titration dosing schema in the PROUD-PV study. Moreover, CMR was observed in two patients and PMR in 29 patients (63.0%), contributing to an MR rate of 67.3% in this study. It also appeared that most patients with a greater reduction of the JAK2V617F allelic burden experienced CHR. This is consistent with the notion that the potential disease-modifying effect exerted by ropeginterferon alfa-2b is at least in part associated with a decrease in the JAK2V617F allelic burden. JAK2V617F allelic burden is an important biomarker in PV. Patients with a higher JAK2V617 VAF had an increased risk of thrombotic complications. JAK2V617F MR in patients with PV or essential thrombocythemia was associated with a lower risk of progression to MF [44, 45]. Therefore, further exploration of the relationship between the efficient reduction of the JAK2V617F allelic burden by ropeginterferon alfa-2b and improved disease-modifying outcomes such as prolonged progression-free or overall survival for patients is required.
Ropeginterferon alfa-2b was well-tolerated and no new safety issues were identified in this study. Most of the AEs were mild or moderate. Grade 4 or 5 AEs were not observed in any patient. Grade 3 AEs were manageable and reversible. The most common AEs were ALT and AST elevations, with only one case of grade 3 ALT elevation, none of which were associated with the occurrence of jaundice or symptoms. One case of patient discontinuation due to AEs was observed. Previously, initial higher doses of PEGylated IFNs needed to be decreased stepwise based on tolerance [46]. However, our study has demonstrated that ropeginterferon treatment can be initiated with a starting dose of 250 µg, which is close to the highest, target dose of 500 µg. The dose of 500 µg can be achieved within 4 weeks of the first treatment. Moreover, depression which was associated with prior IFN-based therapies was not observed in this study. Furthermore, the PK exposures regarding Cmax and AUC were found to increase with the dose. Both parameters exhibited a dose-dependent increase. The mean Cmax at 500 µg in the study was 59.5 ng/mL and the mean AUC was 15,292 ± 5,625.8 h × ng/mL. The higher starting dose regimen appeared to lead to effective plasma exposures associated with good efficacy and tolerability. Thus, the results of this study regarding efficacy, MR, safety, and PK indicate a positive benefit-risk profile with this dosing regimen as a treatment option for patients with PV.
In summary, ropeginterferon alfa-2b at the 250-350-500 µg dosing regimen with a flexibility of dose adjustment according to tolerability appears to show good clinical tolerability and safety, and efficacy with meaningful PK exposures in patients with PV. The results indicate a positive benefit-risk balance in patients treated with ropeginterferon alfa-2b at this dosing regimen, suggesting that the treatment could lead to durable clinical benefits at a greater level with a reasonable safety profile. The participating patients in this study are expected to receive long-term continued treatment to assess progression-free and overall survivals.
Acknowledgments
The authors thank all the participants and study coordinators, nurses, and other investigators involved in this study. We are grateful to the patients and their families.
Financial Disclosure
The study was sponsored and supported by PharmaEssentia Corporation.
Conflict of Interest
SS Suo, RF Fu, ZH Shao, J Bai, SN Chen, MH Duan, H Zhou, N Xu, SJ Zhang, XL Zuo, X Du, L Wang, P Li, XH Zhang, ZJ Xiao, and J Jin have no conflict of interest to disclose. A Qin, O Zagrijtschuk, and T Sato are employees of PharmaEssentia Corporation. DX Wu, WH Shen, W Wang, JJ Zhang, and YN Li are employees of PharmaEssentia Biotech (Beijing) Ltd.
Informed Consent
Written informed consent was obtained from all participating patients.
Author Contributions
Each author listed contributed to the work and takes responsibility for the content. All authors participated in the writing and review process of the manuscript and approved it for publication. J Jin, ZJ Xiao, A Qin, DX Wu, YN Li, JJ Zhang, W Wang, and O Zagrijtschuk designed the study. DX Wu, A Qin, and J Jin wrote the initial draft of the manuscript. SS Suo, RF Fu, ZH Shao, J Bai, SN Chen, MH Duan, H Zhou, N Xu, SJ Zhang, XL Zuo, X Du, L Wang, P Li, XH Zhang, ZJ Xiao, and J Jin enrolled and treated patients on the trial.
Data Availability
The data supporting the findings of this study can be available from PharmaEssentia upon reasonable request after ropeginterferon alfa-2b has acquired marketing approvals in China.
| References | ▴Top |
- Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15.
doi pubmed pmc - Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1(1):97-105.
doi pubmed - Kc D, Falchi L, Verstovsek S. The underappreciated risk of thrombosis and bleeding in patients with myelofibrosis: a review. Ann Hematol. 2017;96(10):1595-1604.
doi pubmed pmc - Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia. 2021;35(12):3339-3351.
doi pubmed pmc - Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(5):1071-1082.
doi pubmed pmc - Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, Bogani C, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21(9):1952-1959.
doi pubmed - Gerds AT, Mesa R, Burke JM, Grunwald MR, Scherber R, Yu J, Hamer-Maansson J, et al. P1062: A real-world evaluation of the association between elevated blood counts and thrombotic events in polycythemia vera: an analysis of data from the REVEAL study. Hemasphere. 2022;6(Suppl):952-953.
- Huang YW, Qin A, Fang J, Wang TF, Tsai CW, Lin KC, Teng CL, et al. Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics and safety in a phase I clinical trial. Br J Clin Pharmacol. 2022;88(5):2396-2407.
doi pubmed - Lin HH, Hsu SJ, Lu SN, Chuang WL, Hsu CW, Chien RN, Yang SS, et al. Ropeginterferon alfa-2b in patients with genotype 1 chronic hepatitis C: pharmacokinetics, safety, and preliminary efficacy. JGH Open. 2021;5(8):929-940.
doi pubmed pmc - Miyachi N, Zagrijtschuk O, Kang L, Yonezu K, Qin A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin Drug Investig. 2021;41(4):391-404.
doi pubmed - Chen CY, Chuang WL, Qin A, Zhang WH, Zhu LY, Zhang GQ, Chen JJ, et al. A phase 3 clinical trial validating the potency and safety of an innovative, extra-long-acting interferon in chronic hepatitis C. JGH Open. 2022;6(11):782-791.
doi pubmed pmc - Them NC, Bagienski K, Berg T, Gisslinger B, Schalling M, Chen D, Buxhofer-Ausch V, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol. 2015;90(4):288-294.
doi pubmed pmc - Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, Wolf D, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126(15):1762-1769.
doi pubmed pmc - Gisslinger H, Grohmann-Izay B, Georgiev P, Skotnicki A, Gercheva-Kyuchukova L, Egyed M, Rossiev V, et al. Final results from PEN-PV study, a single-arm phase 3 trial assessing the ease of self-administrating ropeginterferon alfa-2b using a pre-filled PEN in polycythemia vera patients. Haematologica. 2017;102(s2):816-817.
- Gisslinger H, Buxhofer-Ausch V, Thaler J, Forjan E, Willenbacher E, Wolf D, Kralovics R, et al. Long-term efficacy and safety of ropeginterferon alfa-2b in patients with polycythemia vera—final phase I/II Peginvera study results. Blood. 2018;132(Suppl 1):3030.
- Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, Rossiev V, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196-e208.
doi pubmed - Kiladjian JJ, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, Dulicek P, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia. 2022;36(5):1408-1411.
doi pubmed pmc - Edahiro Y, Ohishi K, Gotoh A, Takenaka K, Shibayama H, Shimizu T, Usuki K, et al. Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. Int J Hematol. 2022;116(2):215-227.
doi pubmed - United States Food & Drug Administration. FDA news release: FDA approves treatment for rare blood disease. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-rare-blood-disease. Accessed 15 December 2021.
- European Medicines Agency. Besremi (ropeginterferon alfa-2b). Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/besremi. Accessed 15 December 2021.
- Qin A, Urbanski RW, Yu L, Ahmed T, Mascarenhas J. An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: an overview of previous and ongoing studies with perspectives on the future. Front Oncol. 2023;13:1109866.
doi pubmed pmc - Verstovsek S, Komatsu N, Gill H, Jin J, Lee SE, Hou HA, Sato T, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022;18(27):2999-3009.
doi pubmed - Huang CE, Wu YY, Hsu CC, Chen YJ, Tsou HY, Li CP, Lai YH, et al. Real-world experience with Ropeginterferon-alpha 2b (Besremi) in Philadelphia-negative myeloproliferative neoplasms. J Formos Med Assoc. 2021;120(2):863-873.
doi pubmed - Huang YW, Hsu CW, Lu SN, Yu ML, Su CW, Su WW, Chien RN, et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol Int. 2020;14(6):997-1008.
doi pubmed pmc - Jin J, Qin A, Zhang L, Shen W, Wang W, Zhang J, Li Y, et al. A phase II trial to assess the efficacy and safety of ropeginterferon alpha-2b in Chinese patients with polycythemia vera. Future Oncol. 2023;19(11):753-761.
doi pubmed - Jin J, Zhang L, Qin A, Wu D, Shao Z, Bai J, Chen S, et al. A new dosing regimen of ropeginterferon alfa-2b is highly effective and tolerable: findings from a phase 2 study in Chinese patients with polycythemia vera. Exp Hematol Oncol. 2023;12(1):55.
doi pubmed pmc - Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H, Kiladijan JJ, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148(6):961-963.
doi pubmed - Schuster SC. Next-generation sequencing transforms today's biology. Nat Methods. 2008;5(1):16-18.
doi pubmed - Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Kiladjian JJ, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829-4833.
doi pubmed - Barosi G, Mesa R, Finazzi G, Harrison C, Kiladjian JJ, Lengfelder E, McMullin MF, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781.
doi pubmed pmc - Huang YW, Tsai CY, Tsai CW, Wang W, Zhang J, Qin A, Teng C, et al. Pharmacokinetics and pharmacodynamics of novel long-acting ropeginterferon alfa-2b in healthy Chinese subjects. Adv Ther. 2021;38(9):4756-4770.
doi pubmed - Buxhofer-Ausch V, Gisslinger H, Thiele J, Gisslinger B, Kvasnicka HM, Mullauer L, Frantal S, et al. Leukocytosis as an important risk factor for arterial thrombosis in WHO-defined early/prefibrotic myelofibrosis: an international study of 264 patients. Am J Hematol. 2012;87(7):669-672.
doi pubmed - Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, De Stefano V, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33.
doi pubmed - Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130(16):1795-1799.
doi pubmed pmc - Rinder HM, Schuster JE, Rinder CS, Wang C, Schweidler HJ, Smith BR. Correlation of thrombosis with increased platelet turnover in thrombocytosis. Blood. 1998;91(4):1288-1294.
pubmed - Moliterno AR, Kaizer H, Reeves BN. JAK2 V617F allele burden in polycythemia vera: burden of proof. Blood. 2023;141(16):1934-1942.
doi pubmed pmc - Stein BL, Oh ST, Berenzon D, Hobbs GS, Kremyanskaya M, Rampal RK, Abboud CN, et al. Polycythemia Vera: An Appraisal of the Biology and Management 10 Years After the Discovery of JAK2 V617F. J Clin Oncol. 2015;33(33):3953-3960.
doi pubmed pmc - Shirane S, Araki M, Morishita S, Edahiro Y, Sunami Y, Hironaka Y, Noguchi M, et al. Consequences of the JAK2V617F allele burden for the prediction of transformation into myelofibrosis from polycythemia vera and essential thrombocythemia. Int J Hematol. 2015;101(2):148-153.
doi pubmed - Abu-Zeinah G, Krichevsky S, Cruz T, Hoberman G, Jaber D, Savage N, Sosner C, et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia. 2021;35(9):2592-2601.
doi pubmed pmc - Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, Dulicek P, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. 2023;37(10):2129-2132.
doi pubmed pmc - Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, Ritchie E, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood. 2019;134(18):1498-1509.
doi pubmed pmc - Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A, Harrison CN, et al. A randomized phase 3 trial of interferon-alpha vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139(19):2931-2941.
doi pubmed pmc - Verger E, Soret-Dulphy J, Maslah N, Roy L, Rey J, Ghrieb Z, Kralovics R, et al. Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo. Blood Cancer J. 2018;8(10):94.
doi pubmed pmc - Guglielmelli P, Loscocco GG, Mannarelli C, Rossi E, Mannelli F, Ramundo F, Coltro G, et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021;11(12):199.
doi pubmed pmc - Guglielmelli P, Mora B, Gesullo F, Mannelli F, Loscocco GG, Signori L, Pessina C, et al. JAK2 V617F molecular response to ruxolitinib in patients with PV and ET is associated with lower risk of progression to secondary myelofibrosis. Blood. 2022;140(Supple 1):1788-1789.
- Quintas-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL, Zhang SJ, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122(6):893-901.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


