| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 13, Number 1-2, April 2024, pages 1-11
Anorectal Infections in Neutropenic Leukemia Patients: A Common Clinical Challenge
Rodrick Babakhanloua, Farhad Ravandi-Kashania, Angel G. Hitab, Dimitrios P. Kontoyiannisc, d
aDepartment of Leukemia, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
bDepartment of Emergency Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
cDivision of Internal Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
dCorresponding Author: Dimitrios P. Kontoyiannis, Division of Internal Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX 77030, USA
Manuscript submitted February 16, 2024, accepted March 29, 2024, published online April 9, 2024
Short title: Anorectal Infections in the Neutropenic Leukemia
doi: https://doi.org/10.14740/jh1251
- Abstract
- Introduction
- Anorectal Infections
- Anorectal Infections in the Neutropenic Leukemia Patients
- Conclusion
- References
| Abstract | ▴Top |
Anorectal infections in neutropenic leukemia patients are a significant and potentially life-threatening complication. The pathogenesis of this condition is not entirely understood and believed to be multifactorial, including mucosal injury as a result of cytotoxic drugs, profound neutropenia and impaired host defense. Establishing an early diagnosis is key and often made clinically on the basis of signs and symptoms, but also from imaging studies demonstrating perianal inflammation or fluid collection. The management of anorectal infections in neutropenic leukemia patients is not straightforward, as there are no well-conducted studies on this entity. This review seeks to provide a framework into the pathophysiology and clinical presentation of anorectal infections in neutropenic leukemia patients, propose a diagnostic approach and to discuss controversies in the management of this condition.
Keywords: Anal abscess; Rectal abscess; Anorectal sepsis; Perianal sepsis; Acute leukemia; Leukemia
| Introduction | ▴Top |
The current approach to treating acute leukemia, including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), incorporates the combination of potent chemotherapeutic agents and targeted molecular therapies with the goal of achieving complete remission (CR) or even cure [1-4]. Patients undergoing treatment for acute leukemia are at risk of life-threatening complications, either from the disease itself or from its therapy [3]. These complications can be either infectious or non-infectious, with a common involvement of the intestinal tract [3]. Severe chemotherapy-induced neutropenia is one complication frequently observed, placing the patient at increased risk of infections [3, 5-8]. Neutropenia is defined as an absolute neutrophil count (ANC) of < 1,500/µL and is being classified as mild, moderate and severe [3, 6-8]. Severe neutropenia, defined as an ANC of < 500/µL, can be the result from intensive cytotoxic chemotherapy during the management of hematological malignancies [3, 7]. In up to 30% of cases, the gastrointestinal tract (GIT) is the source of infection in neutropenic patients, especially if neutropenia is prolonged or severe [6, 9].
Anorectal infections are not uncommon in the neutropenic patients and have been reported to occur in 5-9% of patients with hematological malignancies, with mortality rates ranging from 50% to 78% [6, 10, 11]. Various papers have reported different mortality rates, reflecting differences in the studied leukemia patient populations and their comorbidities, chemotherapy regimens, antibacterial prophylaxis and management approaches of those infections.
This review will discuss the pathophysiology, clinical presentation, assessment and current controversies and challenges in the management of anorectal infections in the neutropenic leukemia patients.
| Anorectal Infections | ▴Top |
Anorectal abscesses are common conditions, which in most cases arise from cryptoglandular infections within the anal canal [12]. Physiologically, anal glands drain into anal crypts at the dentate line via ducts. Blockage of those ducts can result in retention of fecal material, leading to inflammation of the glands and subsequent abscess formation [12]. In 90% of cases, perianal abscesses result from blockage of the ducts, while in the remaining 10%, they occur as a result of immunosuppression, malignancies, inflammatory bowel disease or post radiation [12]. Based on their location, abscesses are classified as intersphincteric, perianal, ischiorectal or supralevator, as illustrated in Figure 1 [12]. While anal abscesses represent the acute phase of disease, fistula formations are the consequences of untreated or complicated abscesses and represent a chronic process [12].
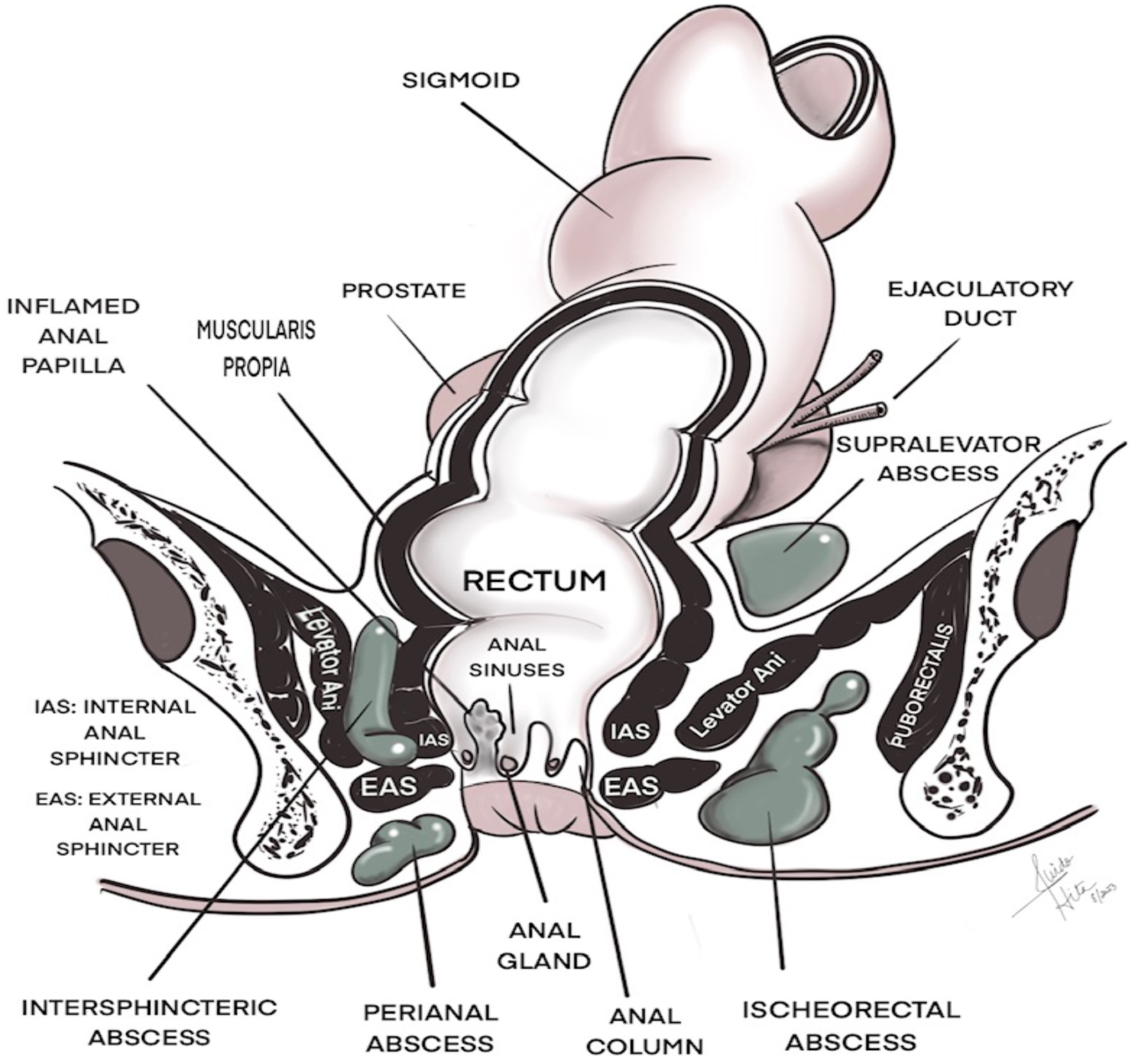 Click for large image | Figure 1. Location of anorectal abscesses. |
| Anorectal Infections in the Neutropenic Leukemia Patients | ▴Top |
Background and pathogenesis
Anorectal infections in neutropenic leukemia patients are not uncommon and represent a potentially fatal complication [13-15]. The pathogenesis of anorectal infections in neutropenic leukemia patients is incompletely understood and probably multifactorial due to the combination of mucosal injury from cytotoxic chemotherapy or constipation-induced trauma, the presence of neutropenia and an impaired local (mucosal) and systemic host defense [3, 11, 16]. One of the most significant risk factors for anorectal infections is profound neutropenia, which can result either as a consequence of high-dose myelosuppressive chemotherapy or the disease itself [3, 13, 14]. Patients with AML generally receive high-dose cytarabine-based chemotherapy with significantly prolonged and profound neutropenia and severe mucositis [17]. Ara-C is known to be associated with acute mucosal injury and necrosis of the mucosal layer, complicated by a delayed regeneration of the epithelial cells, which may be one reason, why patients with AML are more prone to develop anorectal infections [3, 18]. Although a clear association with vincristine and anorectal infections has not been reported in literature in adult leukemia patients, pseudo-obstruction and neurotoxicity have been common adverse effects, especially in the pediatric leukemia population [19]. In addition, although methotrexate-induced mucosal injury is frequent in pediatric patients with ALL, a clear association with methotrexate and anorectal infection in adult patient is not known [20].
Patients with leukemia frequently receive opioids for pain management, which predisposes them to constipation. Furthermore, there is an additional risk of constipation in patients with ALL, who are treated with vincristine. Constipation and repetitive mucosal trauma caused by hard stool are known risk factors for mucosal injury, increasing the risk for anorectal infections [16].
Neutrophils are an essential component of the inflammatory process, the primary response to infections and formation of pus and abscess collection [10, 14]. Thus, a prolonged duration of neutropenia increases the risk of developing perianal sepsis in leukemia patients, while pus collection and abscess formation may be absent due to lack of neutrophils [21-23].
The exact route of entry of pathogens into the perianal region in neutropenic patients remains unclear, but it is believed that while the presence of mucosal injury provides the port of entry of pathogens, neutropenia and the presence of opportunistic infections facilitate the development of anorectal infections [13, 15, 17]. Further risk factors include the presence of comorbidities, such as diabetes mellitus or preexisting anorectal conditions, like hemorrhoids or fissures [16, 22-24].
Most infections are polymicrobial with gram-negative bacilli being the most predominant, followed by gram-positive cocci, anaerobes and Candida species [16, 17, 25]. While perirectal abscess cultures are dominant with gram-positive coccobacilli, gram-negative rods, gut anaerobes and Candida spp., blood cultures have predominantly shown E. coli, Enterococcus spp., Klebsiella, and Pseudomonas [22, 24].
Another possible factor for the development of anorectal infections is the alteration of the gut microbiome, which is important for the maintenance of local and systemic immune tone and immune homeostasis, mucosal integrity and competition that provides colonization resistance against invading pathogens [3]. A low diversity of both oral and gut microbiome has frequently been observed in patients with leukemia, which can be attributed to a prolonged duration of neutropenia as a result of multiple lines of chemotherapy and administration of broad-spectrum antibiotics over a long period of time, which promotes the growth of pathogenic bacteria and gut dysbiosis [26].
Although it has been noted that intestinal diseases often are accompanied by changes in the diversity and abundance of gut microbiota, at this stage the correlation between the gut microbiome and anorectal infections is not fully understood and it is not known whether the impact of specific microbiome compositions predisposes neutropenic patients to the development of anorectal infections [27].
Clinical presentation
In the immunocompetent patients, anorectal infections may present with perirectal pain worse on defecation or urination, urinary retention, rectal discharge or back pain [12].
In the immunosuppressed patients on the other hand, clinical manifestations may differ entirely and are influenced by the severity and duration of neutropenia, the concomitant use of immunosuppressive medications, such as corticosteroids or the presence of comorbidities, such as diabetes mellitus [28].
Since neutrophils are critical in the response of anorectal infections, the clinical presentation in neutropenic patients can be blunted and vague, and patients with prolonged and profound neutropenia may not exhibit the classical signs of anorectal infections. Symptoms usually begin when neutrophil counts nadir around 7 - 14 days after cytotoxic chemotherapy and may include fever, tenesmus, rectal bleeding or purulent discharge, a new onset of perianal pain and discomfort, erythema and induration. However, a fluctuant abscess may not be present due to the low neutrophil count [3, 13, 28]. Perirectal pain accompanied by urinary symptoms, including retention or dysuria, should raise the suspicion of an intersphincteric or supralevator abscess [12]. In addition, the development of fever in a patient with a prior anorectal abscess most likely points to a recurrent infection arising in the anorectal area [29].
Diagnosis
The assessment of neutropenic patients with perianal symptoms can be challenging, as currently there are no “gold-standard” diagnostic criteria and no consensus on the optimal approach. Establishing the diagnosis is currently based on a thorough history taking, physical examination, laboratory tests and radiological findings [12, 13].
Clinical examination can be challenging, since the clinical picture in immunosuppressed patients can be non-specific as a result of profound neutropenia, delaying the establishment of the diagnosis and resulting in a worse outcome [3, 13, 30, 31]. Examination should start with assessing the vital signs to look for the presence of fever, tachycardia, dehydration, hypotension or the presence of toxicity. Inspection of the perineal region may reveal erythema, induration and swelling, but fluctuation may be absent due to low neutrophil counts. Since certain types of abscesses, such as an intersphincteric abscess, may not be visible on inspection, digital rectal examination (DRE) can be helpful in establishing the diagnosis in the immunocompetent patients [12]. However, undertaking DRE in the immunosuppressed patients is currently highly discouraged due to concerns of breaching mucosal barriers and worsening septicemia, despite controversial opinions [15, 16, 28]. Physical examination may also reveal enlarged inguinal lymph nodes.
Laboratory evaluation should assess for the presence of pancytopenia, specifically investigating the degree of neutropenia as well as blood chemistries [3].
Both blood cultures and wound cultures should be obtained prior to initiation of antibiotic therapy. Although most infections are polymicrobial, culture results can help targeting anti-infective therapy.
Radiographic imaging modalities are reliable diagnostic tools for the assessment of anorectal infections and include ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI) [15].
Contrast-enhanced CT scans of the abdomen and pelvis are readily available and the diagnostic tool of choice for the acutely unwell patient presenting to the emergency room. The advantages of CT scans include its lower cost, greater availability, rapid acquisition and excellent resolution [32-34]. CT scans are helpful in identifying abscesses, small amounts of free air and delineating both superficial and deep fascia structures [14, 34, 35]. The use of contrast is preferred over non-contrast examination, as it can help demonstrating small fluid collections, inflammatory changes, abscesses and fistulous tracts [34, 35]. However, the use of contrast is not always feasible due to the presence of renal impairment [32].
While large abscesses can be demonstrated on CT scans, it is important to bear in mind that CT scans lack sensitivity in detecting small perirectal abscesses in the immunosuppressed patients, as in this patient population, abscess formation may be absent [34]. Due to the fact that anorectal infections in the immunocompromised patient may only present with diffuse perianal swelling and edema, this can challenge the accuracy of CT scans in such a setting because of its inferior soft tissue contrast resolution [34-36]. Therefore, MRI scans are the preferred option for the evaluation of anorectal infections in this patient population, as they can establish the pathological anatomy in nearly all forms of anorectal infections with an accuracy of 90%. Moreover, MRI is more accurate for the illustration of soft tissue changes, edema formation, fluid collection and demonstration of complex fistula tracts, although these are less common in patients with hematological malignancies [13, 15, 32, 33]. In T2-weighted sequences, tissue inflammation and abscesses are hyperintense with peripheral enhancement and central diffusion restriction [34]. One disadvantage though is that MRI scans may not be readily available in the acute setting and are time consuming. Moreover, patient selection is crucial, as not all patients are able to undergo MRI due to their body habitus, extensive examination time or preexisting claustrophobia. Given the fact that MRI scans may not be readily available in the acute setting, it is recommended to initially perform a CT scan followed by an MRI at the earliest convenience. If clinical suspicion of anorectal infection exists, treatment should not be delayed based on findings of imaging. If the clinical condition of the patient has not improved despite anti-infective treatment, repeating CT scan 5 - 7 days after initiation of treatment or ideally an MRI is recommended.
The use of ultrasonography in the assessment of anorectal infections in the immunosuppressed patients has limitations. Although ultrasonography is widely available, cheap and non-invasive, it has several disadvantages [37]. The assessment of pathologies of the bowel requires operator experience and expertise and also depends on the patient’s cooperation and body habitus. Moreover, the presence of intraluminal bowel gas can make the visualization of the gut difficult [37].
Endorectal ultrasonography is a highly accurate tool for the assessment of anorectal abscesses and fistulae in the immunocompetent patients. However, its use in the neutropenic leukemia patients should be avoided, since this exam requires the insertion of a rigid probe into the anal canal up to the distal rectum, potentially resulting in a breach of the mucosal barrier, and consequently, worsening septicemia [37, 38].
Therapy
Currently, there is no level-1 evidence to guide clinicians on the best approach in this complex patient population and controversies still surround the optimal management, as there are no randomized controlled studies for that entity. A multidisciplinary approach is recommended in order to determine the optimal treatment strategy, which includes the collaboration between hematologists, gastroenterologists, surgeons and infectious disease specialists [13]. A suggestive approach has been outlined in Figure 2.
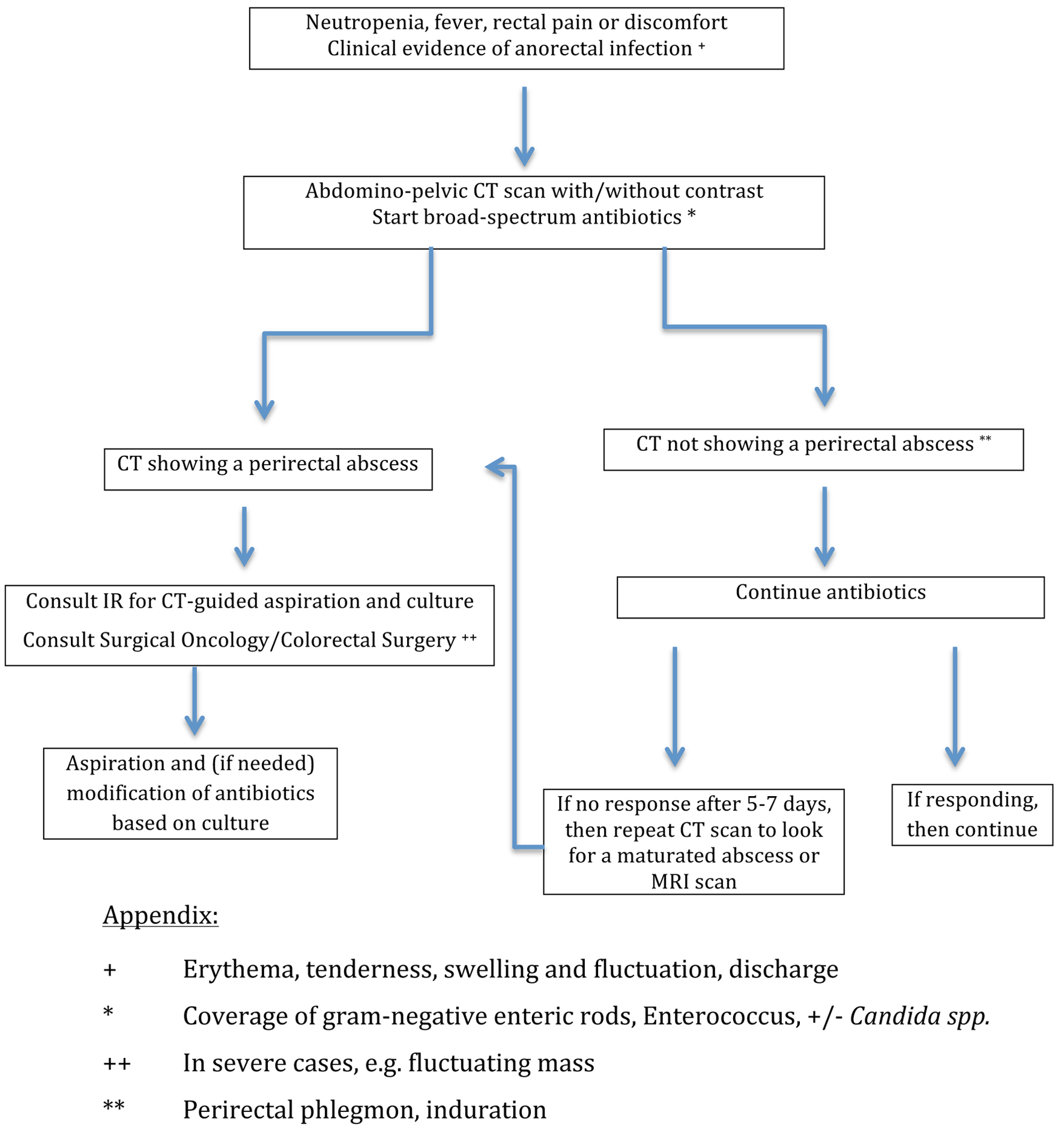 Click for large image | Figure 2. The approach to perirectal abscesses in neutropenic leukemia patients. |
Anti-infective therapy
After having obtained blood cultures, the prompt initiation of empiric antimicrobial therapy is critical, covering likely pathogens that inhabit the anorectal canal, such as gram-positive coccobacilli, gram-negative rods, anaerobic bacteria and possibly Candida species. Since infections in immunosuppressed patients are polymicrobial consisting of potentially resistant pathogens (e.g., if the patient has had prior antibiotic treatments for bacterial infections), the choice of broad-spectrum antimicrobial therapy should be guided by an institution-specific, and ideally, a leukemia-unit specific antibiogram and consideration of comorbidities and recent antibiotic exposure [3, 13].
The IDSA guidelines recommend the prompt initiation of empiric antibiotics and suggest cefepime, a carbapenem or piperacillin-tazobactam and recommend their use for at least the duration of neutropenia or until there are clear signs of marrow recovery [39]. However, those recommendations were made for the management of neutropenia, but do not specifically address anorectal infections.
In cases, where cephalosporins are used as first agent, consideration of adding metronidazole for better coverage of anaerobes should be made [40]. The addition of metronidazole is not necessary if other lactams with excellent anaerobic coverage are used, such as piperacillin-tazobactam or carbapenems [3].
Antibiotics should be continued for the standard length of the infection and until the ANC rises above 500/µL. Once the ANC is greater than 500/µL, they can be switched from intravenous to oral formulations [41]. Important aspects to incorporate regarding the duration and intensity of anti-infective treatment is the net state of immunosuppression (e.g., cytopenia, the use of corticosteroids, active underlying malignant disease and comorbidities) [14].
The use of long-term antibiotics and chemotherapy in the leukemia patient can induce a rapid change of the gut microbiome resulting in dysbiosis and selection of multi-drug resistant bacteria [42]. Alterations of the gut microbiome are linked to an altered immune response of the intestine resulting in intestinal diseases [27]. Currently correlations between the gut microbiome and perianal infections in leukemia patients are not fully understood [27]. And although rectal swabs are a reliable tool to assess the colonic microbiome, their use in assisting in antibiotic selection is not well validated or widely in use [42-44].
Given the anatomical location and the complex perineal flora, fungal co-infections are not uncommon and initiation of antifungal agents covering Candida species, such as echinocandins should strongly be considered in selected patients [3, 39]. The duration of treatment for anorectal infections is not based on quality evidence and should be made on a case-to-case basis based on clinical presentation and laboratory evaluation [3]. The differential diagnosis of anorectal pathologies in neutropenic leukemia patients is broad. In patients with non-healing ulcerations in the perianal area with a poor response to antibiotics, it is important to consider other causes, as outlined in Table 1 [45]. Examples include herpes simplex infections, which often fail to exhibit vesicular lesions due to profound neutropenia or leukemic infiltrations of the perineal area [16, 28, 45].
 Click to view | Table 1. Differential Diagnosis of Non-Healing Perianal Lesions in Cytopenic Patients With Leukemia [28, 45] |
Surgical approach
In immunocompetent patients, surgical interventions varying from incision and drainage, and the creation of a colostomy in certain severe cases, are the standard approach, since without operative intervention the infection might spread into adjacent tissues leading to necrotizing fasciitis [29, 46]. However, in the immunosuppressed leukemia patients, an operative approach is hindered by several factors, including frailty, increasing bleeding tendency and a high risk of subsequent wound infection and poor wound healing [29].
While several studies in literature advocate a surgical approach in the immunosuppressed patients, others recommend a non-operative approach. However, there are marked differences in the study size, patient population, type of malignancies, definition of anorectal infections, the impact of neutrophil counts on the outcome, the duration of neutropenia, the timing and type of surgery and the use of CT-guided fine-needle aspiration, which make a generalized recommendation difficult [10, 11, 16, 21, 22, 24, 25, 30, 31, 46-48].
Those studies of medical with or without surgical approach are summarized in Table 2.
 Click to view | Table 2. Summary of Representative Studies of Medical Management With or Without Surgical Approach in Anorectal Infections in Cancer Patients |
With a better understanding of the pathophysiology of disease and improved supportive care measures, an aggressive surgical approach has largely been avoided, especially in patients with pancytopenia, because of fear of prolonged bleeding, delayed wound healing and possible wound infections postoperatively [29]. Currently, management is individualized and influenced by the level of host compromise, the degree of frailty, presence of comorbidities, the need for further cytotoxic chemotherapy, the degree of pancytopenia, anticipated neutrophil recovery and prognosis, factors that make a conservative approach more favorable [13, 41]. Since in the majority of cases contemporary broad-spectrum antibiotics have been able to control anorectal infections without aggressive surgical intervention, clinicians have reserved open surgical approaches for only severe cases and after neutrophil recovery and formation of a fluctuating mass to facilitate an incision and drainage [16, 22, 33, 41].
In contrast, CT-guided aspiration of the abscess is a commonly used alternative and can serve both diagnostic and therapeutic purposes [49]. This can be either a temporary maneuver to stabilize the patient’s condition before definitive surgery or even result in a complete resolution of the abscess [49].
In some individual cases authors have reported the creation of colostomies, either in form of a loop colostomy or a Hartmann procedure, to divert stool and to allow healing of the anorectal region [21, 48, 50]. However, there are no large studies addressing the benefit of ostomies for the management of anorectal infections in adult neutropenic leukemia patients. Moreover, ostomies can be associated with both short-term and long-term complications, which need to be incorporated in the decision-making [50].
Therapeutic granulocyte transfusions
Anorectal infections in severely neutropenic patients with advanced hematological malignancies can rapidly progress despite adequate anti-infective measures and lead to sepsis and death. In addition, these infections can be indirect contributors to poor outcomes as severe cases can lead to a delay or reduction of subsequent chemotherapy, contributing to leukemia relapse [41, 51-53].
In order to support the immune system with the intention to shorten the duration of neutropenia and to achieve a faster resolution of infections, granulocyte transfusions can support the restoration of granulocyte counts and reduce the risk of infection in this patient population [51, 52].
In a retrospective review of patients with hematological diseases with perianal infections, 22 patients received a total of 148 granulocyte transfusions. Treatment was well tolerated and successfully increased the ANC with an improved 7-day survival in responders [54].
In the setting of allogeneic stem cell transplantation, a prospective, non-randomized trial showed granulocyte transfusions to be effective both as an interventional treatment option in life-threatening infections, but also a suitable option to prevent recurrent infections in high-risk patients [55]. The authors concluded that granulocyte transfusions might improve survival on the short term and enable patients to undergo subsequent cytotoxic therapies [55].
However, literature is limited in this regard and larger randomized controlled studies are needed to define the optimal strategy for its use, including the timing and duration of its application and the patient population that will benefit most from this approach. Moreover, the cost-effectiveness and the risk-versus-benefit ratio of this strategy need to be evaluated [51, 56].
Prophylaxis
Recurrence of perianal abscesses in patients with acute leukemia is not uncommon, since multiple cycles of chemotherapy are administered in order to achieve CR or even cure [29]. Abscesses frequently occur after induction and re-induction chemotherapy or even consolidation therapy as a consequence of severe neutropenia [29]. The recurrence rate of anorectal infections has been reported to be as high as 73%, and patients, who have had one episode of perianal abscess, have a 10-fold increased risk of a subsequent perianal infection [23, 29].
Hence, prophylactic measures may reduce the rate of recurrence, especially in high-risk patients, such as those, where neutropenia is expected to last longer than 7 days, who have frailty, comorbidities, history of perirectal infection and aggressive leukemia [41, 57, 58].
General anti-infective recommendations for high-risk patients include antibacterial prophylaxis with fluoroquinolones, antiviral prophylaxis with acyclovir or valacyclovir to prevent reactivation of the herpes virus and anti-fungal prophylaxis [39, 41]. Antifungal prophylaxis with a triazole is indicated in patients with an ANC < 100/µL lasting more than seven days. This degree of neutropenia increases the risk of invasive Candida infections and the use of oral triazole antifungal drug (voriconazole, posaconazole) is effective in preventing Candida infections [39, 41]. However, whether prophylactic anti-infective treatment prevents recurrent anorectal infections is not known and remains to be evaluated.
For patients, whose risk of febrile neutropenia exceeds 20%, the IDSA guidelines recommend the prophylactic use of colony-stimulating factors [39]. Further indications include patients > 65 years of age, those with poor performance status, poor nutritional status, previous episodes of febrile neutropenia and serious comorbidities [41, 58, 59]. Currently there are no studies illustrating the benefit of colony-stimulating factors or granulocyte transfusion as prophylaxis in patients with history of severe anorectal infection with septicemia. One possible concern with the use of growth factors is that in patients with excess blasts, there is a greater risk of further increasing the percentage of blasts [41].
In addition, supportive measures such as hygiene, rigorous education for avoidance of constipation (e.g. hydration, physical activity, avoidance of narcotics for pain control) can help reducing the risk of recurrence [16].
Pain management
The anal canal is divided into an upper and a lower part by the dentate line, which is the fusion site of the embryonic entoderm and ectoderm. This landmark is the junction between two origins of nerve and blood supply, but also venous and lymphatic drainage [12]. While the intestine above the dentate line is innervated by the sympathetic and parasympathetic nervous system and is insensitive to pain, the intestine below the dentate line is innervated by the somatic nervous system and is highly sensitive to pain [12].
The intensity of pain associated with perianal abscesses depends on their location. While intersphincteric and perianal abscesses have limited space to expand and may cause worsening anal and pelvic pain within a short period of time, supralevator and ischiorectal abscesses may remain asymptomatic for a longer period. Pain management of anorectal abscesses poses a challenge to the clinician, as options are limited. Acetaminophen inhibits central prostaglandin synthesis. Despite its reasonable efficacy and favorable side effect profile, it may not sufficiently treat pain in cancer patients. Moreover, caution is advised in patients with liver disease, as the drug is metabolized in the liver [59].
Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the synthesis of prostaglandins through the synthesis of cyclooxygenase enzyme. The most common side effect of NSAIDs is gastrointestinal bleeding, which can be devastating in patients with pancytopenia. Renal and hepatic toxicity are potential side effects, especially with long-term use [59]. Therefore, NSAIDs are not an ideal option.
Opioids are typically necessary to control pain and act on central nervous system receptors to modulate pain. Options include codeine, oxycodone and hydrocodone. Significant side effects include nausea, vomiting, sedation, significant risk of dependence, and moreover, constipation, which subsequently can worsen repetitive trauma of the anal canal [59]. Therefore, any use of opioids should always be combined with laxatives to avoid constipation or even obstruction. If the pain in this patient population cannot be adequately controlled, consulting the pain team for further advise and options is recommended.
Implications for subsequent chemotherapy
The decision for further chemotherapy depends on the treatment intent. In a palliative situation it is important to evaluate risks versus benefits of further chemotherapy by assessing the impact of chemotherapy-related toxicity and loss of quality of life versus small prolongation of life and lack of achieving and maintaining remission [60]. In such situations, a delay of the next cycle of chemotherapy, or modifications of the dose and regimen should be considered in order to avoid further episodes of neutropenic sepsis [60].
In situations with a curative intent, it is very important to keep both the dose and interval of chemotherapy and its schedule [60]. In such situations, in order to accelerate recovery of neutrophil counts before initiation of the next cycle, the use of granulocyte colony-stimulating factor (G-CSF) may be indicated [60].
Moreover, the use of granulocyte transfusions might provide an alternative option in the prophylactic setting with the aim to prevent infections and to enable patients to undergo subsequent cytotoxic therapies [55].
| Conclusion | ▴Top |
Anorectal infections are a common and important complication in neutropenic leukemia patients with a high recurrence rate and a serious cause of morbidity and mortality. The exact pathogenesis and predisposing factors remain undefined and many questions regarding the management of this condition remain unanswered, including the choice and duration of anti-infective therapy, ideal prophylactic measures, implications for subsequent chemotherapy or the optimal surgical intervention, as outlined in Table 3.
 Click to view | Table 3. Ongoing Questions Regarding Risk Factors and Management of Anorectal Infections in Neutropenic Patients With Leukemia |
Existing literature is not helpful regarding the ideal approach and is due to different study designs and different patient populations. While studies in the past advocated a radical surgical approach, improvement in supportive care in this patient population over the last decade has led to a reduced infection-related morbidity and mortality. Thus, aggressive surgical intervention has been deemphasized and should await recovery of neutrophil counts in selected patients with severe anorectal infections. If surgical intervention is warranted, but not feasible, CT-guided procedures have emerged the preferred source control approach.
Collaboration between hematologists, gastroenterologists, surgeons and infectious disease specialists is recommended to outline the best possible approach. More research is needed to improve understanding of the pathogenesis of this condition in order to outline preventative measures, and to better define treatment strategies, including the optimal timing to return to oncological treatment.
Acknowledgments
The authors thank Dr Hita for providing the illustration in Figure 1.
Financial Disclosure
None to declare.
Conflict of Interest
RB has no conflict of interest. AGH has no conflict of interest. FR receives honoraria, research funds, consulting fees from Astellas, AstraZeneca, Xenocor, Prelude, Abbvie, Novartis, Syos, Amgen, Celgene/BMS and Astex/Taiho. DPK reports honoraria and research support from Gilead Sciences and Astellas, Inc., received consultant fees from Astellas Pharma, Merck, and Gilead Sciences, and is a member of the Data Review Committee of Cidara Therapeutics, AbbVie, and the Mycoses Study Group.
Author Contributions
RB wrote the manuscript. FR revised and approved the manuscript. AGH provided the illustrations and approved the manuscript. DK corrected, updated and approved the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; ANC: absolute neutrophil count; CR: complete remission; CT: computed tomography; DRE: digital rectal examination; G-CSF: granulocyte colony-stimulating factor; GIT: gastrointestinal tract; MRI: magnetic resonance imaging; NSAIDs: non-steroidal anti-inflammatory drugs
| References | ▴Top |
- Babakhanlou R, Ravandi-Kashani F. SOHO State of the Art Updates and Next Questions |The role of maintenance therapy in acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2023;23(1):1-7.
doi pubmed - Babakhanlou R, Ravandi-Kashani F. Non-intensive acute myeloid leukemia therapies for older patients. Expert Rev Hematol. 2023;16(3):171-180.
doi pubmed - Babakhanlou R, Ravandi-Kashani F, Kontoyiannis DP. Neutropenic enterocolitis: an uncommon, but fearsome complication of leukemia. J Hematol. 2023;12(2):59-65.
doi pubmed pmc - Babakhanlou R, DiNardo C, Borthakur G. IDH2 mutations in acute myeloid leukemia. Leuk Lymphoma. 2023;64(11):1733-1741.
doi pubmed - Gorschluter M, Glasmacher A, Hahn C, Leutner C, Marklein G, Remig J, Schmidt-Wolf IG, et al. Severe abdominal infections in neutropenic patients. Cancer Invest. 2001;19(7):669-677.
doi pubmed - White MG, Morgan RB, Drazer MW, Eng OS. Gastrointestinal surgical emergencies in the neutropenic immunocompromised patient. J Gastrointest Surg. 2021;25(12):3258-3264.
doi pubmed pmc - Siebert M, Lucas N, Gelli M, Sourrouille I, Benhaim L, Faron M, Micol JB, et al. Acute abdominal complications in deeply neutropenic onco-hematology patients: a retrospective series of 105 cases. World J Surg. 2022;46(10):2389-2398.
doi pubmed - Abu-Sbeih H, Ali FS, Chen E, Mallepally N, Luo W, Lu Y, Foo WC, et al. Neutropenic Enterocolitis: Clinical Features and Outcomes. Dis Colon Rectum. 2020;63(3):381-388.
doi pubmed - Perazzoli C, Parra RS, Feitosa MR, Sfoggia E, Simoes BP, Rocha JJR, Feres O. A novel severity score index for febrile neutropenic patients with colorectal diseases. Gastroenterol Res Pract. 2019;2019:4175960.
doi pubmed pmc - Morcos B, Amarin R, Abu Sba A, Al-Ramahi R, Abu Alrub Z, Salhab M. Contemporary management of perianal conditions in febrile neutropenic patients. Eur J Surg Oncol. 2013;39(4):404-407.
doi pubmed - Badgwell BD, Chang GJ, Rodriguez-Bigas MA, Smith K, Lupo PJ, Frankowski RF, Delclos G, et al. Management and outcomes of anorectal infection in the cancer patient. Ann Surg Oncol. 2009;16(10):2752-2758.
doi pubmed - Babakhanlou R. Anorectal Disorders. In: David SS (ed). Clinical Pathways in Emergency Medicine. 2016. p. 371-392. Springer India.
- Sullivan PS, Moreno C, Shaib WL. Management of anorectal and intra-abdominal infections in the neutropenic cancer patient. Curr Probl Cancer. 2015;39(5):274-286.
doi pubmed - Baker B, Al-Salman M, Daoud F. Management of acute perianal sepsis in neutropenic patients with hematological malignancy. Tech Coloproctol. 2014;18(4):327-333.
doi pubmed - Ashkar C, Britto M, Carne P, Cheung W, Mirbagheri N. Perianal sepsis in neutropaenic patients with haematological malignancies: the role of magnetic resonance imaging and surgery. ANZ J Surg. 2020;90(9):1642-1646.
doi pubmed - North JH, Jr., Weber TK, Rodriguez-Bigas MA, Meropol NJ, Petrelli NJ. The management of infectious and noninfectious anorectal complications in patients with leukemia. J Am Coll Surg. 1996;183(4):322-328.
pubmed - Chen CY, Cheng A, Huang SY, Sheng WH, Liu JH, Ko BS, Yao M, et al. Clinical and microbiological characteristics of perianal infections in adult patients with acute leukemia. PLoS One. 2013;8(4):e60624.
doi pubmed pmc - Ebert EC, Hagspiel KD. Gastrointestinal manifestations of leukemia. Journal of Gastroenterology and Hepatology. 2012;27(3):458-463.
- Diezi M, Nydegger A, Di Paolo ER, Kuchler H, Beck-Popovic M. Vincristine and intestinal pseudo-obstruction in children: report of 5 cases, literature review, and suggested management. J Pediatr Hematol Oncol. 2010;32(4):e126-130.
doi pubmed - Pashankar FD, Season JH, McNamara J, Pashankar DS. Acute constipation in children receiving chemotherapy for cancer. J Pediatr Hematol Oncol. 2011;33(7):e300-303.
doi pubmed - Buyukasik Y, Ozcebe OI, Sayinalp N, Haznedaroglu IC, Altundag OO, Ozdemir O, Dundar S. Perianal infections in patients with leukemia: importance of the course of neutrophil count. Dis Colon Rectum. 1998;41(1):81-85.
doi pubmed - Lehrnbecher T, Marshall D, Gao C, Chanock SJ. A second look at anorectal infections in cancer patients in a large cancer institute: the success of early intervention with antibiotics and surgery. Infection. 2002;30(5):272-276.
doi pubmed - Solmaz S, Korur A, Gereklioglu C, Asma S, Buyukkurt N, Kasar M, Yeral M, et al. Anorectal complications during neutropenic period in patients with hematologic diseases. Mediterr J Hematol Infect Dis. 2016;8(1):e2016019.
doi pubmed pmc - Loureiro RV, Borges VP, Tome AL, Bernardes CF, Silva MJ, Bettencourt MJ. Anorectal complications in patients with haematological malignancies. Eur J Gastroenterol Hepatol. 2018;30(7):722-726.
doi pubmed - Barnes SG, Sattler FR, Ballard JO. Perirectal infections in acute leukemia. Improved survival after incision and debridement. Ann Intern Med. 1984;100(4):515-518.
doi pubmed - Galloway-Pena JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, Chemaly RF, et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122(14):2186-2196.
doi pubmed pmc - Yin H, Luo B, Wang Q, Hong Z, Chen H, Shen L, Shen B, et al. Differences in gut microbiota between healthy individuals and patients with perianal abscess before and after surgery. Mediators Inflamm. 2023;2023:1165916.
doi pubmed pmc - Granwehr B, Kontoyiannis DP. The evolving landscape of gastrointestinal infections in neutropenic patients. Oncology (Williston Park). 2015;29(8):590.
pubmed - Chang H, Kuo MC, Tang TC, Lin TL, Wu JH, Hung YS, Wang PN. Clinical features and recurrence pattern of perianal abscess in patients with acute myeloid leukemia. Acta Haematol. 2017;138(1):10-13.
doi pubmed - Corfitsen MT, Hansen CP, Christensen TH, Kaae HH. Anorectal abscesses in immunosuppressed patients. Eur J Surg. 1992;158(1):51-53.
pubmed - Boddie AW, Jr., Bines SD. Management of acute rectal problems in leukemic patients. J Surg Oncol. 1986;33(1):53-56.
doi pubmed - Khati NJ, Sondel Lewis N, Frazier AA, Obias V, Zeman RK, Hill MC. CT of acute perianal abscesses and infected fistulae: a pictorial essay. Emerg Radiol. 2015;22(3):329-335.
doi pubmed - Haliloglu N, Gulpinar B, Ozkavukcu E, Erden A. Typical MR imaging findings of perianal infections in patients with hematologic malignancies. Eur J Radiol. 2017;93:284-288.
doi pubmed - Guniganti P, Lewis S, Rosen A, Connolly S, Raptis C, Mellnick V. Imaging of acute anorectal conditions with CT and MRI. Abdom Radiol (NY). 2017;42(2):403-422.
doi pubmed - Caliste X, Nazir S, Goode T, Street JH, 3rd, Hockstein M, McArthur K, Trankiem CT, et al. Sensitivity of computed tomography in detection of perirectal abscess. Am Surg. 2011;77(2):166-168.
pubmed - Plumb AA, Halligan S, Bhatnagar G, Taylor SA. Perianal Sepsis in Hematologic Malignancy: MR Imaging Appearances and Distinction from Cryptoglandular Infection in Immunocompetent Patients. Radiology. 2015;276(1):147-155.
doi pubmed - Bor R, Fabian A, Szepes Z. Role of ultrasound in colorectal diseases. World J Gastroenterol. 2016;22(43):9477-9487.
doi pubmed pmc - Visscher AP, Felt-Bersma RJ. Endoanal ultrasound in perianal fistulae and abscesses. Ultrasound Q. 2015;31(2):130-137.
doi pubmed - Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad, II, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-93.
doi pubmed - Babakhanlou R, Larkin K. The value of Metronidazole in the management of acute radiation proctitis. Scand J Gastroenterol. 2021;56(4):422-423.
doi pubmed - Keng MK, Sekeres MA. Febrile neutropenia in hematologic malignancies. Curr Hematol Malig Rep. 2013;8(4):370-378.
doi pubmed - Turner G, O'Grady M, Hudson D, Morgan X, Frizelle F, Purcell R. Rectal swabs are a reliable method of assessing the colonic microbiome. Int J Med Microbiol. 2022;312(2):151549.
doi pubmed - Biehl LM, Garzetti D, Farowski F, Ring D, Koeppel MB, Rohde H, Schafhausen P, et al. Usability of rectal swabs for microbiome sampling in a cohort study of hematological and oncological patients. PLoS One. 2019;14(4):e0215428.
doi pubmed pmc - Schlebusch S, Graham RMA, Jennison AV, Lassig-Smith MM, Harris PNA, Lipman J, P OC, et al. Standard rectal swabs as a surrogate sample for gut microbiome monitoring in intensive care. BMC Microbiol. 2022;22(1):99.
doi pubmed pmc - Ruymbeke H, Geldof J, De Looze D, Denis MA, De Schepper H, Dewint P, Gijsen I, et al. Secondary anal fissures: a pain in the a*. Acta Gastroenterol Belg. 2023;86(1):58-67.
doi pubmed - Glenn J, Cotton D, Wesley R, Pizzo P. Anorectal infections in patients with malignant diseases. Rev Infect Dis. 1988;10(1):42-52.
doi pubmed - Carlson GW, Ferguson CM, Amerson JR. Perianal infections in acute leukemia. Second place winner: Conrad Jobst Award. Am Surg. 1988;54(12):693-695.
pubmed - Grewal H, Guillem JG, Quan SHQ et al. Anorectal disease in neutropenic leukemia patients. Operative vs. non-operative management. Diseases of the Colon and Rectum. 1994:37:1095-1099.
- Wallace MJ, Chin KW, Fletcher TB, Bakal CW, Cardella JF, Grassi CJ, Grizzard JD, et al. Quality improvement guidelines for percutaneous drainage/aspiration of abscess and fluid collections. J Vasc Interv Radiol. 2010;21(4):431-435.
doi pubmed - Babakhanlou R, Larkin K, Hita AG, Stroh J, Yeung SC. Stoma-related complications and emergencies. Int J Emerg Med. 2022;15(1):17.
doi pubmed pmc - Cherif H, Axdorph U, Kalin M, Bjorkholm M. Clinical experience of granulocyte transfusion in the management of neutropenic patients with haematological malignancies and severe infection. Scand J Infect Dis. 2013;45(2):112-116.
doi pubmed - Safdar A, Rodriguez G, Zuniga J, Al Akhrass F, Pande A. Use of healthy-donor granulocyte transfusions to treat infections in neutropenic patients with myeloid or lymphoid neoplasms: experience in 74 patients treated with 373 granulocyte transfusions. Acta Haematol. 2014;131(1):50-58.
doi pubmed pmc - Kumar AJ, Gimotty PA, Gelfand JM, Buck G, Rowe JM, Goldstone AH, Fielding A, et al. Delays in postremission chemotherapy for Philadelphia chromosome negative acute lymphoblastic leukemia are associated with inferior outcomes in patients who undergo allogeneic transplant: An analysis from ECOG 2993/MRC UK ALLXII. Am J Hematol. 2016;91(11):1107-1112.
doi pubmed pmc - Roman D, Kantarjian HM, Cortes JE et al. Granulocyte transfusions for neutropenic patients with perirectal and perineal infections. Blood. 2018;132(1):2544.
- Mousset S, Hermann S, Klein SA, Bialleck H, Duchscherer M, Bomke B, Wassmann B, et al. Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Ann Hematol. 2005;84(11):734-741.
doi pubmed - Strauss RG. Therapeutic granulocyte transfusions: neutropenic patients with acute leukemia continue to need them - why are definitive evidence-based practice guidelines elusive? Transfusion. 2019;59(1):6-8.
doi pubmed - Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, Kuderer NM, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(6):794-810.
doi pubmed - de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F, Group EGW. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v252-256.
doi pubmed - Stamos MJ, Hicks TC. Pain management in anorectal surgery. Seminars in Colon and Rectal Surgery. 2006;17:125-130.
- Cameron D. Management of chemotherapy-associated febrile neutropenia. Br J Cancer. 2009;101(Suppl 1):S18-22.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


