| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 5, October 2024, pages 224-228
Scedosporium Brain Abscess: A Rare and Fatal Drawback of Bruton Tyrosine Kinase Inhibitor Therapy
Matteo Dalmazzoa, Melissa Padrinia, Sofia Camerloa, Giorgio Rosatia, Tiziano Tommaso Busanaa, Paolo Nicolia, Fabio Perottob, Luca Daviccoc, d, Pietro Caironic, d, Marco De Gobbia, Alessandro Morottia, e
aDepartment of Clinical and Biological Sciences, University of Turin, AOU San Luigi Gonzaga, 10043 Orbassano, Italy
bDepartment of Radiology, University of Turin, AOU San Luigi Gonzaga, 10043 Orbassano, Italy
cDepartment of Anesthesia and Critical Care, University of Turin, AOU San Luigi Gonzaga, 10043 Orbassano, Italy
dDepartment of Oncology, University of Turin, AOU San Luigi Gonzaga, 10043 Orbassano, Italy
eCorresponding Author: Alessandro Morotti, Department of Clinical and Biological Sciences, University of Turin, AOU San Luigi Gonzaga, 10043 Orbassano, Italy
Manuscript submitted March 15, 2024, accepted June 19, 2024, published online October 21, 2024
Short title: Scedosporium Brain Abscess
doi: https://doi.org/10.14740/jh1263
| Abstract | ▴Top |
The patient described in this case report was admitted to the San Luigi Hospital in Turin for confusion, drowsiness, and buccal and eye deviation. An acute neurological disease was suspected. He was affected by chronic lymphocytic leukemia (CLL) on active treatment with the novel Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib. Other comorbidities included type II diabetes mellitus, arterial hypertension, and nonalcoholic steatohepatitis. Imaging exams showed multiple brain lesions, which appeared to be of infectious-inflammatory origin. Consequently, therapy with acalabrutinib was withheld. The patient was later transferred to the intensive care unit, because of worsening neurological conditions. The definite diagnosis of fungal abscess was obtained through a stereotactic biopsy of the widest brain lesion. Microbiological tests confirmed Scedosporium spp. as the etiological agent. Once a detailed antibiogram had been obtained, voriconazole therapy was started. However, the patient’s clinical conditions decayed rapidly and he later died of neurological complications. BTKis represent a milestone in the treatment of CLL; however, little is known about how these molecules act on the immune system. Fungal brain abscesses are rare conditions more commonly seen in heavily immunocompromised patients, such as those affected by acquired immune deficiency syndrome, after bone marrow transplant or treatment for acute leukemia. Whether or not therapy with BTKis can favor opportunistic fungal infections is still a matter of debate. Various reports of Aspergillosis infections developing after therapy with ibrutinib exist. Evidence does suggest that an iatrogenic impairment in the innate immune system could favor these infections. In addition, the patient’s comorbidities, such as diabetes mellitus and advancing hematological disease, might create the ideal breeding ground for these microorganisms.
Keywords: Bruton tyrosine kinase inhibitors; Abscess; Opportunistic fungal infection; Immunosuppression
| Introduction | ▴Top |
Patients affected by chronic lymphocytic leukemia (CLL) are at increased risk of contracting infections sustained by opportunistic microorganisms.
Since the introduction of Bruton tyrosine kinase inhibitor (BTKi), a number of invasive fungal infections have been reported in treated patients. Cases of Aspergillosis have in fact been described in patients on therapy with ibrutinib, whereas data regarding acalabrutinib are still scarce [1-4]. Acalabrutinib targets and binds to BTK more specifically than its predecessor, as a result, it causes a lower rate of side effects which are more commonly associated with ibrutinib, such as atrial fibrillation, bleeding, and thrombocytopenia. Still, very little is known about how this latest generation BTKi acts on the immune system and how it contributes to the immunosuppressive state already created by CLL [5].
In this case report, we described a patient affected by relapsed CLL on therapy with acalabrutinib. He was diagnosed with CLL in March 2015 and was treated with a total of six cycles of fludarabine-cyclophosphamide-rituximab (FCR) scheme chemotherapy, which he tolerated well, with a complete hematological response by August 2015. There were no complications during this first treatment cycle, the patient continued attending annual follow-up visits, and no signs of disease relapse were reported.
In February 2022, a bone marrow biopsy was performed after the appearance of enlarged axillary lymph nodes and leukocytosis associated with thrombocytopenia on his routine blood tests. The biopsy demonstrated a relapsed CLL, and computed tomography (CT) scan showed diffuse lymphadenopathies in the abdomen and mediastinal region and splenomegaly. As a result, therapy with second-generation BTKi acalabrutinib was started. A CT scan performed after 1 year confirmed an important reduction in size of his diffuse lymphadenopathies, and also his clinical conditions and blood count improved. At the beginnging of May 2023, he was diagnosed with bacterial pneumonia and was started on antibiotic therapy with ceftriaxone, and acalabrutinib was not discontinued. No other infectious complications were reported during the treatment.
The patient’s comorbidities included type II diabetes mellitus, on therapy with metformin and dapagliflozin, hypercholesterolemia, nonalcoholic steatohepatitis (NASH), and arterial hypertension.
At the time of hospital admission, he was on therapy with acalabrutinib.
He presented to the emergency department on May 21, 2023 for neurological symptoms which were later found to be caused by a brain abscess.
| Case Report | ▴Top |
Investigations
A 68-year-old male presented to the emergency department of the San Luigi Hospital in Turin for confusion, drowsiness, and buccal and eye deviation. His medical record showed that he was affected by CLL and had previously been initiated on acalabrutinib.
Physical examination was carried out: he appeared alert, well-oriented in space and time, and cooperative, and his Glasgow coma scale (GCS) was 15. His pupils were isochoric and isocyclic, with normal pupillary light reflex. His eyesight was deviated to the right, with limited eye movement to the left during fixation, he did not have ptosis or visual impairment, and the oral fissure was slightly deviated to the left. Mingazzini I sign was positive on his left arm which was also hypoesthesic, whereas Mingazzini II was normal. He did not have any signs of dysmetria and his plantar reflex was normal. The patient did not present meningeal signs (no neck stiffness, Brudzinski and Kernig signs were negative), or headache and emesis.
Pulmonary examination did not reveal abnormal respiratory sounds, and cardiac and abdominal examinations were normal. Routine blood tests were requested and showed: white blood cell (WBC) 6,330/µL (4,500 - 11,000/µL) (neutrophils 4,420/µL (1,900 - 8,000/µL); lymphocytes 1,110/µL (900 - 5,200/µL)), Hb 13.9 g/dL (13 - 17 g/dL), platelet count 194,000/µL (150,000 - 450,000/µL), normal kidney and liver function, IgG 502 mg/dL (750 - 1,560 mg/dL), IgA 106 mg/dL (80 - 450 mg/dL), IgM 15 mg/dL (40 - 300 mg/dL), C-reactive protein 0.89 mg/dL (< 0.80 mg/dL), procalcitonin (PCT) 0.02 ng/mL (< 0.5 ng/mL), prothrombin time (PT) 1.35 (0.80 - 1.20 INR), activated partial thromboplastin time (aPTT) 30.9 s (22.00 - 34.00 s), and fibrinogen 379 mg/dL (150 - 450 mg/dL).
Suspecting a primarily neurological disease, a head CT scan was performed: the images showed an hypodense area 24 mm wide with surrounding edema occupying the left peri-trigonal area; a second lesion was described in the controlateral frontal region and a third in the right basal ganglia. Given the worsening peri-lesional edema and the consequent symptoms of intracranial hypertension, a neurological consult was sought: the patient was started on anti-edema therapy with IV mannitol. He also underwent an electroencephalogram (EEG) scan, which did not document signs of epileptic fits.
Concomitantly, a chest CT scan was requested and showed multiple consolidated areas in the lungs with a tree-in-bud pattern, compatible with a fungal infection. However, serological tests for galactomannan, (1-3)-beta-D-glucan, and toxoplasmosis, which were later performed, were negative.
He was later admitted to the Internal Medicine/Hematology Ward of the San Luigi Hospital.
Diagnosis
Since an infectious etiology was suspected, therapy with acalabrutinib was withheld. The patient was started on empirical antibiotic and antifungal therapy, first with piperacillin/tazobactam 4.5 g tid which was switched to meropenem 2 g tid, linezolid 600 mg bid, and liposomial amphotericin B.
During the hospital stay, a magnetic resonance imaging (MRI) scan was performed. The exam confirmed the presence of multiple brain lesions, with an appearance and radiological pattern suggestive of an infectious-inflammatory etiology (Fig. 1). Following a neurological consult, an MRI spectroscopic scan was executed, which confirmed the lesion’s infectious origin.
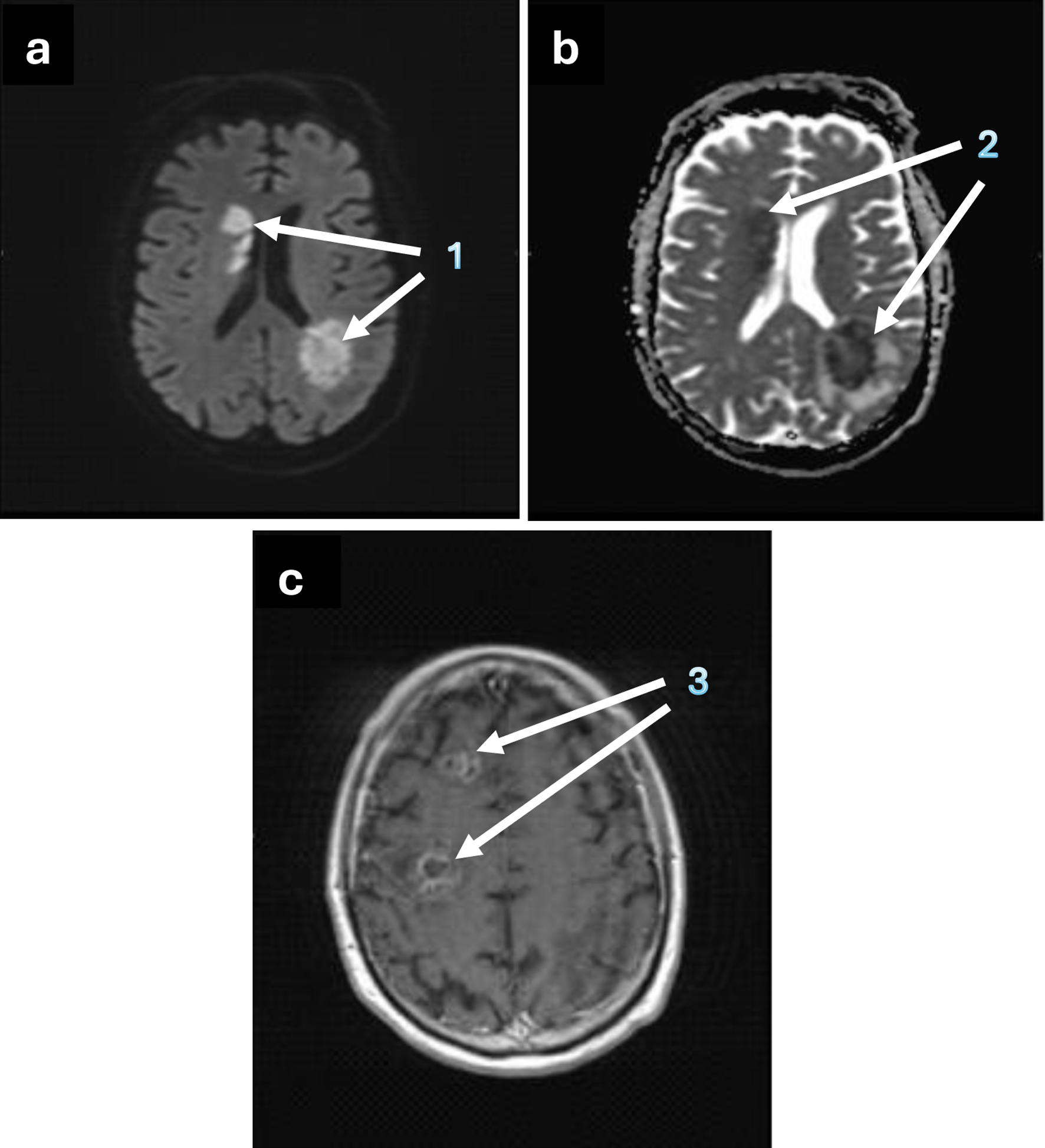 Click for large image | Figure 1. Brain MRI showing the axial view of the abscessual lesions. (a) 1 - hyperintense nodular lesions, compatible with abscessual lesions, on DWI mapping; (b) 2 - abscessual lesions with an hypointense appearance on ADC mapping; (c) 3 - nodular lesions, with contrast enhancement, ring enhancement and peri-lesional oedema after paramagnetic contrast agent injection, on T1-weighted image. ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging; MRI: magnetic resonance imaging. |
However, despite the anti-edema therapy, the patient’s neurological status became gradually worse, and he appeared drowsy, spatially and temporally disoriented. Consequently, he was transferred to the hospital’s intensive care unit.
Treatment and outcomes
Afterward, a stereotactic biopsy was performed on the widest brain lesion. The histological result confirmed that the abscess was of fungal origin, with Scedospoprium spp. being identified as the etiological agent. A detailed antibiogram was requested and antifungal therapy with IV voriconazole was started empirically.
The bioptic procedure was complicated by an intracranial hemorrhage, which extended to the area adjacent to the biopsied lesion and the homolateral cerebral ventricle. The patient therefore underwent a surgical evacuation of the intracerebral hemorrhage. Nonetheless, in the following days, he presented with worsening neurological symptoms, emesis, and facial clonus, and a head CT scan demonstrated a diffuse subarachnoid hemorrhage. A new neurosurgical consult was sought, but no further surgical indications were given. A new head CT scan was planned for the following day to monitor the extent of the hemorrhage; however, the patient’s clinical conditions rapidly decayed, and he became unresponsive and later died.
| Discussion | ▴Top |
To our knowledge, very few studies have investigated the risk of contracting opportunistic infections in patients treated with a BTKi.
Immunocompromised individuals are at increased risk of developing invasive fungal infections. Favoring conditions include undergoing high-dose chemotherapy for hematological malignancies, allogeneic stem cell transplantation, prolonged immunosuppression, persistent neutropenia, corticosteroid therapy, and acquired immune deficiency syndrome. However, even if these conditions are satisfied, infections by Scedosporium remain very rare. Scedosporium is a ubiquitous mold, whose entry route in the human body is commonly via the upper airways. From the respiratory tract, it may spread to other sites, including the central nervous system (CNS), a localization associated with a very poor prognosis. Therapeutic options include voriconazole, posaconazole and liposomal amphotericin B [6].
Pharmacological inhibition of BTK, as caused by acalabrutinib, alters B-cell function and the adaptive immune system, resulting in an acquired state of immunosuppression. A similar effect could be expected in a “natural” BTK inactivation. Bruton’s agammaglobulinemia is an inherited condition characterized by the mutation of the BTK gene, as a result, B cells do not mature properly and their action is impaired. Those affected are at high risk of contracting infections, these however are mainly sustained by viruses and capsulated bacteria [7].
The reason for increased susceptibility to fungal infections could be sought in the involvement of different cellular types, for instance, those belonging to the myeloid-macrophage lineage. Evidence does suggest that BTK is also expressed on neutrophils and macrophages, both part of the innate immune system, which represents the first line of defense against microorganisms like fungi. In these cells, BTK activates a molecular pathway responsible for neutrophil-macrophage chemotaxis, oxidative burst activation, and microbial phagocytosis [8]. Interestingly, a condition known as chronic granulomatous disease, which is characterized by the inability of phagocytic cells to destroy microorganisms because of an inherited defect, poses those affected at a greater risk of developing invasive fungal infections [9].
The artificial inhibition of BTK might affect neutrophils and macrophages and their ability to efficiently contrast these microbes in a similar way to what occurs in the inherited condition [10].
In addition, the combination with the progressing hematological disease could represent an even greater risk factor for contracting invasive infections.
Furthermore, the individual’s baseline characteristics are particularly relevant: the patient in this case report was affected by type II diabetes mellitus, a known risk factor for fungal infections. Upon hospital admission, he did not present with neutropenia and he had not recently received prolonged steroid therapy. However, the hematological disease had relapsed after the first treatment cycle, resulting in an even more impaired immune system and higher susceptibility to infectious events.
Based on current guidelines, once the patient had been started on acalabrutinib, he had not been given anti-fungal prophylaxis. In fact, in the eventuality of starting anti-fungal treatment or prophylaxis, an issue could be represented by the pharmacological interactions: azoles inhibit cytocrome P450, which metabolizes acalabrutinib [11]. For this reason, their concomitant use should be avoided, as it could result in the increased bioavailability of acalabrutinib [12, 13].
In the case of this patient, acalabrutinib was withheld as soon as he was admitted to the hospital and an infectious etiology had been suspected. The fungal abscess was biopsied, in order to define a diagnosis, obtain an antibiogram, and start a tailored anti-fungal therapy. Nevertheless, given the infection’s very poor prognosis and the patient’s decaying general conditions, the therapy was not successful and he later died of neurological complications.
Learning points
BTKis currently represent an important therapeutic option for the treatment of CLL. Acalabrutinib offers a more selective and potent inhibition of BTK, while causing a lesser degree of side effects, which are more commonly associated with ibrutinib. However, it contributes to a state of immunosuppression which may already have been generated by the hematological disease.
We believe that, before BTKi administration, each patient’s individual characteristics and risk factors should be kept carefully into consideration, so as to maximize the therapeutic benefits and minimize the probability of contracting opportunistic infections. Additionally, careful clinical monitoring for prompt diagnosis of fungal infections should be carried out regularly.
To our knowledge, this is one of the few existing reports describing an opportunistic infection by Scedosporium spp. in a patient on treatment with acalabrutinib. Further studies are needed to ascertain a possible link between targeted molecular therapies and the resulting increased infectious risk.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
MD and MP wrote the manuscript; MD, MP, GR, SC, TTB, PN, MDG, and AM evaluated the patients at the Division of Internal Medicine - Hematology; FP assessed radiological images; PC evaluated the patients at the ICU. MDG and AM reviewed manuscript and data.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BTKi: Bruton tyrosine kinase inhibitor; CLL: chronic lymphocytic leukemia; FCR: fludarabine-cyclophosphamide-rituximab; NASH: nonalcoholic steatohepatitis
| References | ▴Top |
- Marchesini G, Nadali G, Facchinelli D, Candoni A, Cattaneo C, Laurenti L, Fanci R, et al. Infections in patients with lymphoproliferative diseases treated with targeted agents: SEIFEM multicentric retrospective study. Br J Haematol. 2021;193(2):316-324.
doi pubmed pmc - Faisal MS, Shaikh H, Khattab A, Albrethsen M, Fazal S. Cerebral aspergillosis in a patient on ibrutinib therapy-A predisposition not to overlook. J Oncol Pharm Pract. 2019;25(6):1486-1490.
doi pubmed - Gaye E, Le Bot A, Talarmin JP, Le Calloch R, Belaz S, Dupont M, Tattevin P. Cerebral aspergillosis: An emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med Mal Infect. 2018;48(4):294-297.
doi pubmed - Reynolds G, Slavin M, Teh BW. Ibrutinib and invasive fungal infections: the known, the unknown and the known unknowns. Leuk Lymphoma. 2020;61(10):2292-2294.
doi pubmed - Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332.
doi pubmed pmc - Seidel D, Meissner A, Lackner M, Piepenbrock E, Salmanton-Garcia J, Stecher M, Mellinghoff S, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope((R)). Crit Rev Microbiol. 2019;45(1):1-21.
doi pubmed - Corneth OBJ, Klein Wolterink RGJ, Hendriks RW. BTK signaling in B cell differentiation and autoimmunity. Curr Top Microbiol Immunol. 2016;393:67-105.
doi pubmed - Lionakis MS, Levitz SM. Host control of fungal infections: lessons from basic studies and human cohorts. Annu Rev Immunol. 2018;36:157-191.
doi pubmed - Yu HH, Yang YH, Chiang BL. Chronic granulomatous disease: a comprehensive review. Clin Rev Allergy Immunol. 2021;61(2):101-113.
doi pubmed - Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, Redelman-Sidi G. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67(5):687-692.
doi pubmed pmc - Chen L, Li C, Bai H, Li L, Chen W. Use of modeling and simulation to predict the influence of triazole antifungal agents on the pharmacokinetics of zanubrutinib and acalabrutinib. Front Pharmacol. 2022;13:960186.
doi pubmed pmc - Wierda WG, Brown J, Abramson JS, Awan F, Bilgrami SF, Bociek G, Brander D, et al. NCCN Guidelines(R) insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, Version 3.2022. J Natl Compr Canc Netw. 2022;20(6):622-634.
doi pubmed - Criscuolo M, Fracchiolla N, Farina F, Verga L, Pagano L, Busca A. A review of prophylactic regimens to prevent invasive fungal infections in hematology patients undergoing chemotherapy or stem cell transplantation. Expert Rev Hematol. 2023;16(12):963-980.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


