| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 2, June 2014, pages 54-56
Heterozygous Factor V Leiden R506Q Mutation Causing a Life-Threatening Bilateral Pulmonary Embolism in a Young Man With Influenza A Pneumonitis
Abdelkarim Wanessa, b, Ashraf Elghula
aInternal Medicine, Mafraq Hospital, Abu Dhabi, UAE
bCorresponding author: Abdelkarim Waness, Department of Medicine, Mafraq Hospital, PO Box 2951, Abu Dhabi, UAE
Manuscript accepted for publication February 25, 2014
Short title: Heterozygous Factor V Leiden
doi: https://doi.org/10.14740/jh127w
| Abstract | ▴Top |
Factor V Leiden (FVL) single R506Q mutation is an inherited familial condition that predisposes to increased risk of thrombophilia. This risk is lower in the heterozygous mutation form compared to the homozygous one. The pathologic clotting might occur at any age group and potentially in various sites of the body vasculature. We present a case of heterozygous form of FVL single R506Q mutation causing life-threatening bilateral pulmonary emboli in a previously healthy young male who suffered severe case of influenza A pneumonitis.
Keywords: Factor V Leiden; Single R506Q mutation; Pulmonary emboli; Pleural effusion; Pericardial effusion; Influenza A; Pneumonitis
| Introduction | ▴Top |
Factor V Leiden (FVL) mutation is the most common inherited blood clotting disorder. The spectrum of this inheritance can range from the more severe homozygous form to the less aggressive heterozygous one. Nonetheless, all carriers of this mutation are prone to having thromboembolic events that can be life-threatening.
| Case Report | ▴Top |
A 35-year-old, previously healthy male was admitted with 7-day history of fever and productive cough unsuccessfully treated with outpatient moxifloxacin. He is an occasional hubbly-bubbly user and denied recent travel or contact with sick individuals. No other personal of family have medical conditions. When evaluated, he was tachypneic with following vital signs: heart rate was 110 bpm, respiratory rate was 22 per minute, blood pressure was 139/86 mm Hg, temperature was 39.6 °C and oxygen saturation was 88% (room air). Subsequent arterial blood gas (room air) revealed: pH 7.43, PCO2 42 mm Hg; PO2 50 mm Hg.
He had diffuse anteroposterior crackles on auscultation. Labs: WBC 1.97 × 109 (range 4.5 - 10), AST 63 IU/L (range 0 - 32), ALT 64 IU/L (range < 31), the rest of his tests were unremarkable. Chest X-ray showed right middle lobe infiltrate. The patient was started on oxygen, broad coverage intravenous antibiotics, and subcutaneous prophylactic heparin and transferred to the intensive care unit. He quickly deteriorated into acute respiratory distress syndrome (ARDS) with respiratory failure requiring mechanical ventilation. Oseltamivir was added to his therapeutic regimen when serology came back positive for influenza A. After 17 days of mechanical ventilation, the patient was extubated and transferred to the ward for further care. Two days later, he became acutely distressed again with severe dyspnea and hypoxemia. A chest CT tomography (CT scan) evidenced extensive bilateral pulmonary artery emboli with stigmata of pneumonia, pleural and pericardial effusions (Fig. 1). The patient was switched to therapeutic low molecular heparin with subsequent bridging to warfarin. His thrombophilia profile identified FVL single R506Q mutation. After 43 days of hospitalization, the patient was discharged home in stable condition. He continues to do well in the outpatient clinic with controlled anticoagulation.
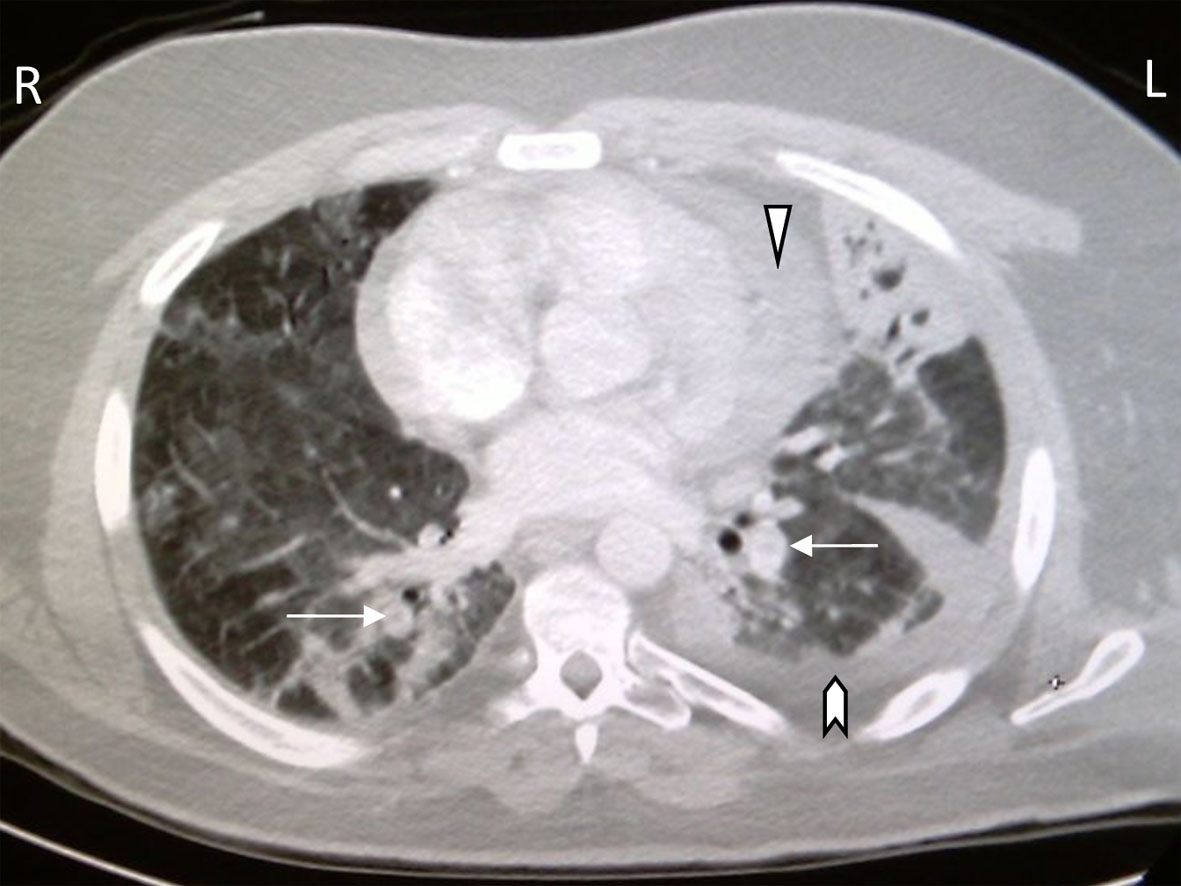 Click for large image | Figure 1. CT chest with bilateral pulmonary artery filling defects (arrows), pericardial effusion (arrowhead), pleural effusions (chevron), pulmonary ground glass appearance and left lung consolidation with air bronchograms. |
| Discussion | ▴Top |
Inherited thrombophilia is caused by a variety of coagulation disturbances including genetic mutations and clotting factors deficiencies. FVL, a genetic mutation named after a Dutch city where it was discovered in 1994, is the leading cause of venous thromboembolism. It results from the substitution of a single nucleotide causing an R506Q mutation. This will cause factor V to be resistant to inactivation by protein C [1]. The high prevalence of FVL mutation in certain ethnic groups such as Northern Europeans is well established. In the Middle East, there is evidence that this mutation is highly prevalent in countries such as Lebanon and Jordan (14.4% and 21.8% respectively) [2, 3]. Venous thrombus formation is dependent upon two important factors: first is the type of mutation inheritance, and secondly the presence of other thrombotic risks. Three types of inheritance are identified: homozygosity, pseudo-homozygosity and heterozygosity [4]. The initial condition carries a much higher thromboembolic risk than the latter. Patients with FVL single R506Q mutation are at a much higher thrombotic risk when facing conditions such as pregnancy, contraceptive use, physical inactivity and airplane travel [5]. Individuals carrying FVL mutation can suffer thrombotic events in various circulatory sites in any age group. Indeed, fetuses with this mutation are occasionally diagnosed with prenatal intrauterine stroke by ultrasonography. The diagnosis is clinically confirmed post-delivery [6]. The spectrum of thromboembolic sites is extremely wide. It can be as common as lower extremity deep venous thrombosis [7] or rare recurrent central retinal vein thromboembolic showering [8]. Some authors recommend testing for FVL mutation in women experiencing recurrent unexplained miscarriages [9]. Our patient had two risk factors stacked against him: first being a heterozygous FVL R506Q mutation carrier, and second enduring a protracted hospitalization. He was able to recover in a dramatic fashion however. Because of the thromboembolic recurrence risk, he was advised to remain on life-long warfarin therapy. Further, he was advised to test his family members for possible discovery of a hidden FVL mutation. Primary prophylactic anticoagulation treatment, for unaffected relatives, is not advised because of increasing potential for bleeding complications [10].
Conclusions
1) Heterozygous FVL single R506Q mutation can be asymptomatic but still carries a considerable risk for thromboembolic events. 2) Other thrombotic risk factors, such as prolonged hospitalization, increase such risk. 3) Physicians should have a high index of suspicion for venous thromboembolism in young healthy individuals. 4) Life-long anticoagulation and genetic counseling must be undertaken in a selective and measured way.
Conflict of Interest
None reported.
Funding Source
Nil.
Consent Form
Obtained.
| References | ▴Top |
- Rosendorff A, Dorfman DM. Activated protein C resistance and factor V Leiden: a review. Arch Pathol Lab Med. 2007;131(6):866-871.
pubmed - Kreidy R, Irani-Hakime N. Is thrombophilia a major risk factor for deep vein thrombosis of the lower extremities among Lebanese patients? Vasc Health Risk Manag. 2009;5:627-633.
doi pubmed - Nusier MK, Radaideh AM, Ababneh NA, Qaqish BM, Alzoubi R, Khader Y, Mersa JY, et al. Prevalence of factor V G1691A (Leiden) and prothrombin G20210A polymorphisms among apparently healthy Jordanians. Neuro Endocrinol Lett. 2007;28(5):699-703.
pubmed - Castoldi E, Rosing J. Factor V Leiden: a disorder of factor V anticoagulant function. Curr Opin Hematol. 2004;11(3):176-181.
doi - Pabinger I, Grafenhofer H. Thrombosis during pregnancy: risk factors, diagnosis and treatment. Pathophysiol Haemost Thromb. 2002;32(5-6):322-324.
doi pubmed - Verdu A, Cazorla MR, Moreno JC, Casado LF. Prenatal stroke in a neonate heterozygous for factor V Leiden mutation. Brain Dev. 2005;27(6):451-454.
doi pubmed - Birewar S, Thomas M, McHale MS. DVT: Factor V Leiden, a case report. S D J Med. 2003;56(6):225-227.
pubmed - Duic J, Gveric-Krecak V. [Recurrent incomplete central retinal vein occlusion in a patient with hereditary thrombophilia]. Acta Med Croatica. 2006;60(2):171-174.
pubmed - Mahjoub T, Mtiraoui N, Tamim H, Hizem S, Finan RR, Nsiri B, Almawi WY. Association between adverse pregnancy outcomes and maternal factor V G1691A (Leiden) and prothrombin G20210A genotypes in women with a history of recurrent idiopathic miscarriages. Am J Hematol. 2005;80(1):12-19.
doi pubmed - Evaluation of Genomic Applications in P, Prevention Working G. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


