| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 13, Number 5, October 2024, pages 216-223
Transformation of Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma to Histiocytic/Dendritic Cell Sarcoma
Jennifer Caia, b, e , David Fernandez-Hazourya, e, Gene Yoshikawac, e, Amani Minjaa, Hehua Huanga, Andrew Hwangc, Xin Qinga, d, f
aDepartment of Pathology, Harbor-UCLA Medical Center, Torrance, CA 90502, USA
bUniversity of California at Irvine, Irvine, CA, USA
cDivision of Hematology and Medical Oncology, Department of Medicine, Harbor-UCLA Medical Center, Torrance, CA, USA
dDavid Geffen School of Medicine at UCLA, Los Angeles, CA, USA
eThese authors contributed equally to the study.
fCorresponding Author: Xin Qing, Hematology and Flow Cytometry Laboratories, Department of Pathology, Harbor-UCLA Medical Center, Torrance, CA 90502, USA
Manuscript submitted June 26, 2024, accepted August 5, 2024, published online September 16, 2024
Short title: CLL/SLL to Histiocytic/Dendritic Cell Sarcoma
doi: https://doi.org/10.14740/jh1310
| Abstract | ▴Top |
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) transforming into a more aggressive lymphoma (i.e., Richter syndrome) is well documented in the literature. In recent years, transdifferentiation of CLL/SLL to histiocytic/dendritic/Langerhans cell sarcomas has also been reported. We hereby describe a case of a 55-year-old female who was incidentally diagnosed with CLL after presenting to the hospital for symptoms of undiagnosed rheumatoid arthritis. At the time of presentation, CLL was stage 1, and the patient was placed on observation. Eight years after being diagnosed with CLL, and after several treatment modalities for her rheumatoid arthritis, the patient re-presented with progression of adenopathy, intermittent fevers, 5-pound weight loss, and worsening respiratory status requiring airway management. Computed tomography (CT) imaging revealed a soft tissue mass in the nasopharynx, lingual tonsillar hypertrophy with airway compromise, and bulky cervical, supraclavicular, and axillary lymphadenopathy. A biopsy of an enlarged cervical lymph node yielded a diagnosis of histiocytic/dendritic cell sarcoma favoring interdigitating dendritic cell sarcoma, likely representing transdifferentiation from CLL/SLL, of which there are no standard of care treatment guidelines. The patient was treated with ifosfamide, carboplatin, and etoposide (ICE) for three cycles, followed by rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) in combination with zanubrutinib. She then underwent haploidentical hematopoietic stem cell transplantation. At the time of the making of this manuscript, the patient was 45 days post-transplant without any notable complications.
Keywords: Chronic lymphocytic leukemia; Small lymphocytic leukemia; Histiocytic/dendritic cell sarcoma; Histiocytic sarcoma; Interdigitating dendritic cell sarcoma; Transformation; Transdifferentiation
| Introduction | ▴Top |
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a common mature B-cell neoplasm consisting of monomorphic small mature B cells with characteristic immunophenotype (CD5 positive, CD23 positive, CD19 positive, CD20 dim, FMC-7 negative, and strongly positive for CD200). Although CLL and SLL are the same disease, the designation of CLL or SLL depends on the disease site, with SLL primarily affecting the lymph nodes, spleen, or other extramedullary organs, and CLL being used for disease manifesting in the peripheral blood with at least 5 × 109/L monoclonal B cells. Patients with less than 5 × 109/L CLL-like cells in the blood and without lymph node, spleen, or other extramedullary involvement are considered to have monoclonal B-cell lymphocytosis. Occasionally, CLL/SLL can transform into a more aggressive lymphoma (i.e., Richter syndrome) among which diffuse large B-cell lymphoma is the most common type. Recently, rare cases of CLL/SLL with clonally related histiocytic/dendritic cell sarcoma [1-3] or neuroendocrine carcinoma [4] were reported, providing evidence of transdifferentiation although the exact relationship between CLL/SLL and histiocytic/dendritic cell sarcoma or neuroendocrine carcinoma remains unknown.
In this report, we describe another unique case of histiocytic/dendritic cell sarcoma developed in a 55-year-old African American female who had a history of CLL/SLL.
| Case Report | ▴Top |
Investigations
The patient is a 55-year-old African American female with a past medical history of anemia and migraine headaches who initially presented to our hospital 8 years before the development of histiocytic/dendritic cell sarcoma with a chief complaint of right wrist pain and swelling. Workup at the time revealed a new diagnosis of seropositive rheumatoid arthritis as well as an absolute lymphocytosis of 9.6 × 103/mm3. Flow cytometry was performed on the peripheral blood. It demonstrated a kappa-restricted B-cell population (88% of total lymphocytes) expressing CD5, CD19, variable CD20, CD23, and variable FMC-7 without co-expression of CD10 or CD38, consistent with CLL. This was initially staged as Rai stage 1 without splenomegaly, hepatomegaly, significant anemia, or thrombocytopenia. Lymphadenopathy was only detectable by computed tomography (CT) scan with lymph nodes in the 1 cm range. The patient was asymptomatic and, thus, was placed on observation. Rheumatoid arthritis was initially managed with hydroxychloroquine, meloxicam, methotrexate, and prednisone. In the year following diagnosis, the patient received a hysterectomy for leiomyoma of the uterus. In the years following diagnosis and intermittent non-compliance with rheumatoid arthritis management, hepatitis B core immunoglobulin M (IgM) was detected. The patient was managed with tenofovir, rituximab infusions, and sulfasalazine.
Eight years after the initial diagnosis of CLL and standard of care monitoring, the patient presented with a progression of lymphadenopathy, intermittent fevers, 5-pound (lb) weight loss, and worsening respiratory status requiring airway management. CT imaging revealed a soft tissue mass in the nasopharynx representing enlarged adenoidal tissue with diffuse palatine and lingual tonsillar hypertrophy with airway narrowing and bulky cervical, supraclavicular, and axillary lymphadenopathy. A positron emission tomography-computed tomography (PET/CT) scan revealed intense 18F-fluoro-2-deoxy-D-glucose (FDG) uptake within the nasal-pharyngeal mass (maximum standardized uptake values (SUVmax) 18.7) and level IIB cervical chain lymph nodes (SUVmax 20.7), and bulky conglomerate of FDG-avid lymph nodes throughout the axillary, periaortic/retroperitoneal, and iliac areas. A completed blood count showed moderate leukocytosis (WBC 40.7 × 103/mm3, normal range 4.5 - 10.0 × 103/mm3; 100-cell manual differential: neutrophils 24, lymphocytes 75, monocyte 1), mild hypochromic-macrocytic anemia (hemoglobin (Hb) 11.8 g/dL, normal range 12.0 - 14.6 g/dL; mean corpuscular volume (MCV) 97.6 fL, normal range 82.0 - 97.0 fL; mean corpuscular hemoglobin concentration (MCHC) 31.1 g/dL, normal range 32.0 - 35.0 g/dL), and normal platelet count (311 × 103/mm3, normal range 160 - 360 × 103/mm3) with increased large/giant platelets (mean platelet volume (MPV) 12.9 fL, normal range 7.0 - 11.0 fL). A review of the peripheral blood smear revealed numerous atypical lymphocytes consistent with persistent CLL. She was tested negative for human immunodeficiency virus, hepatitis A, hepatitis B, hepatitis C, coronavirus disease 2019, influenza A, influenza B, respiratory syncytial virus, QuantiFERON-TB Gold, and direct antiglobulin tests. Additional blood test results are summarized in Table 1. CLL prognosis panel performed at Quest Diagnostics Incorporated showed 87% of lymphoid cells with CD19 positivity. The CD19-positive cells showed 1% positivity for ZAP70 and 20% for CD38. Fluorescence in situ hybridization (FISH) was negative for trisomy 12, deletions of 6q, ATM (11q22.3), 13q14, and TP53 (17p13.1). Chromosomal analysis revealed a normal karyotype. Beta-2 microglobulin was 2.27 mg/L (normal range: ≤ 2.51 mg/L). The patient expressed immunoglobulin heavy-chain variable region gene (IgHV)3-21 gene family with unmutated IgHV status. The expression of IgHV3-21 gene family is associated with poor outcome in CLL, irrespective of the mutation status [5]. These overall findings were consistent with unfavorable prognostic features in CLL. At the time of presentation, the top clinical differential for lymphadenopathy was a transformation of CLL to an aggressive lymphoma, i.e., Richter transformation.
 Click to view | Table 1. Blood Test Results at Harbor-UCLA Medical Center at Presentation of Progressive Lymphadenopathy |
Diagnosis
An ultrasound-guided core biopsy of an enlarged lymph node in the posterior triangle of the right neck was performed. The hematoxylin and eosin (H&E) stained histologic sections showed lymphoid tissue with many paler areas (Fig. 1). The paler areas contained sheets of atypical histiocytic/dendritic cells with irregular nuclear contours and prominent/distinct nucleoli (Fig. 2). Occasional mitotic figures were found, including atypical ones (Fig. 2a). Small mature lymphocytes and scattered eosinophils were admixed (Fig. 2b). By immunohistochemistry (IHC, Table 2, Fig. 3), the histiocytic/dendritic cells were positive for CD4, CD45, CD68 (granular and Golgi pattern), CD163 (small subset), BCL6, cyclin D1, lysozyme, and S-100, weakly positive for OCT-2 and BCL2 (subset), and were negative for CD1a, CD3, CD5, CD10, CD20, CD21, CD23, CD30, CD79a, ALK1, BOB.1, MUM1, PAX-5, and cytokeratin AE1/AE3. Ki67 IHC stain showed a high proliferation index. The morphologic and immunohistochemical features were consistent with histiocytic/dendritic sarcoma with differential diagnoses including histiocytic sarcoma and interdigitating dendritic cell sarcoma. S-100 expression can help to differentiate these two entities. According to the current World Health Organization classification, S-100 expression in histiocytic sarcoma is often weak and focal while neoplastic cells of interdigitating dendritic cell sarcoma consistently express S-100 protein. Nonetheless, histiocytic sarcoma and interdigitating dendritic cell sarcoma may show overlap or hybrid features, defying precise classification, especially in small biopsies as in our cases. In the present case, the histiocytic/dendritic cells were diffusely S-100 positive, but some cells showed weak staining. Given the diffuse S-100 positivity in the submitted specimen, interdigitating dendritic cell sarcoma was favored. The background small lymphocytes were composed of many small T-cells (CD3 and CD5 positive) with a small focus of CLL/SLL (B-cell markers, CD5, and CD23 positive) (Fig. 4). The overall findings may represent histiocytic/dendritic cell sarcoma, which could have transdifferentiated from CLL/SLL. TEMPUS next-generation sequencing (NGS) (Tempus Lab, Inc., Chicago, IL, USA) was performed on the lymph node biopsy paraffin block and the peripheral blood for further molecular characterization of the histiocytic/dendritic cell sarcoma and the CLL, as well as their clonal relationship. NGS of the lymph node sample revealed ALK p.T1151M splice region variant - gain of function (GOF) (15.5%), KRAS p.G13D missense variant (exon 2) - GOF (13.0%), RBM10 p.K707* stop gain - loss of function (LOF) (6.1%), and SPEN p.Q3355* stop gain - LOF (6.1%), as well as overexpression of NFKB1, NFKB2, and MAP2K1 by RNA sequencing. No gene rearrangements or reportable altered splicing events were identified. NGS of the peripheral blood revealed ALK p.T1151M splice region variant - GOF (4.0%), TP53 p.Y163C missense variant - LOF (0.4%), KRAS p.G13D missense variant (exon 2) - GOF (0.4%), CCNE1 p.A214V missense variant - unknown significance (46.5%), NF1 p.A706V missense variant - unknown significance (0.5%), and ATM p.L2452R missense variant - unknown significance (0.3%). No currently approved targeted therapies were reported on either sample.
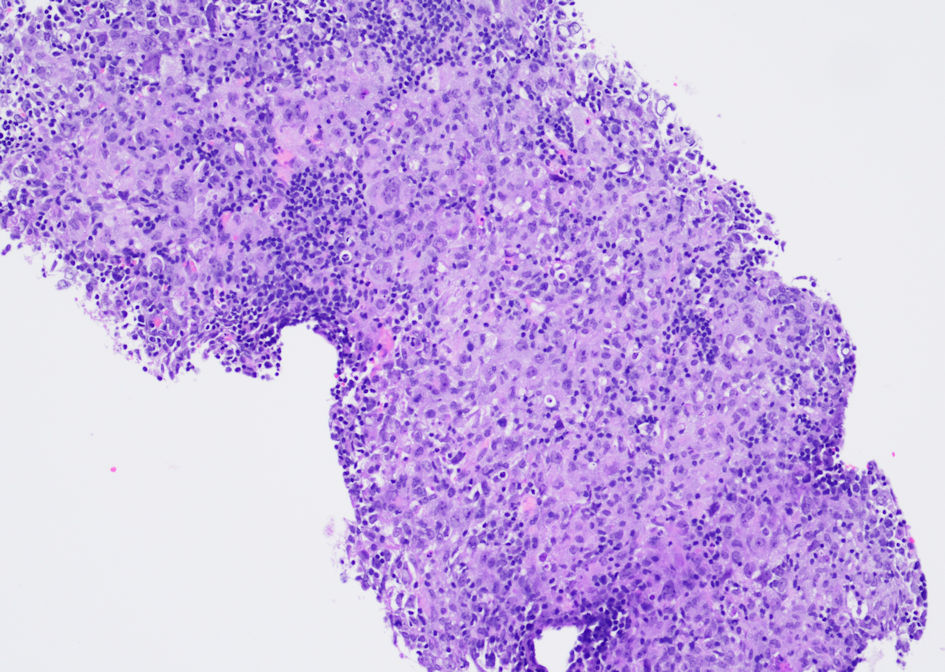 Click for large image | Figure 1. Photomicrograph of the ultrasound-guided core biopsy of an enlarged lymph node in the posterior triangle of the right neck shows lymphoid tissue with many paler areas. (H&E stain, original magnification, × 100). H&E: hematoxylin and eosin. |
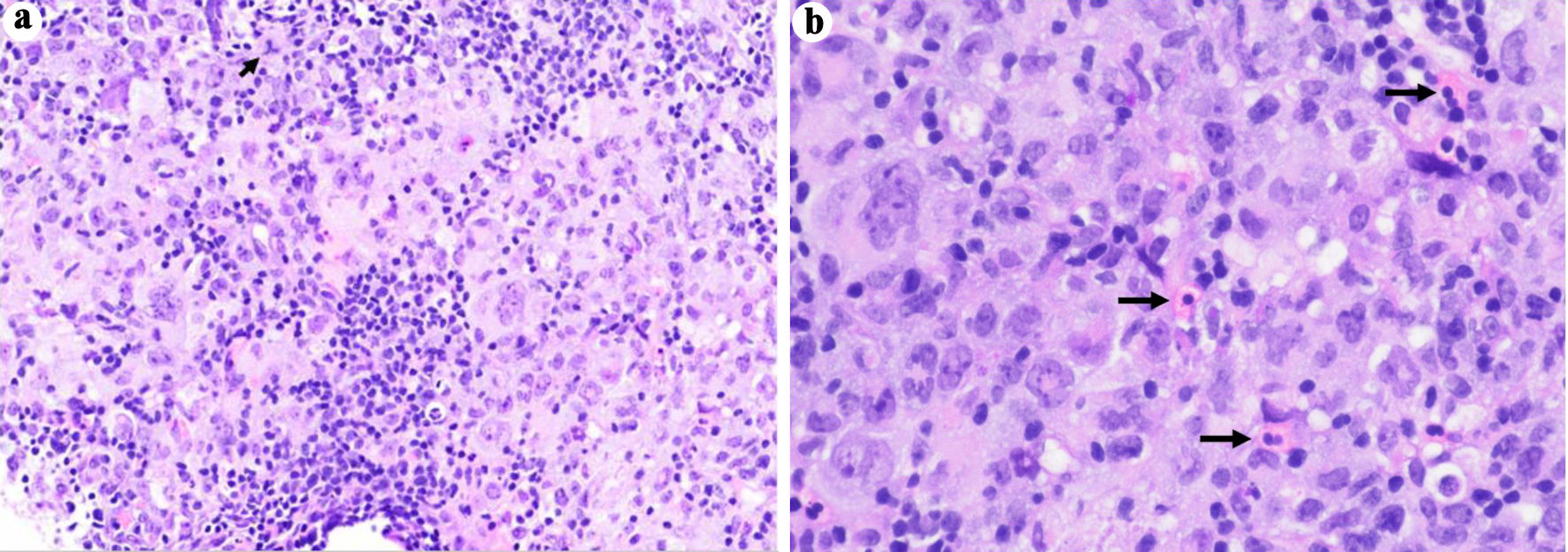 Click for large image | Figure 2. Photomicrographs of the ultrasound-guided core biopsy of an enlarged lymph node in the posterior triangle of the right neck. The paler areas contain sheets of atypical histiocytic/dendritic cells with irregular nuclear contours and prominent/distinct nucleoli. There are occasional mitotic figures, including atypical ones (a, short arrow). Small mature lymphocytes and scattered eosinophils (b, long arrows) are admixed (H&E stain, original magnification, (a) × 200, (b) × 400). H&E: hematoxylin and eosin. |
 Click to view | Table 2. Immunologic Characterization of the Histiocytic/Dendritic Cells by Immunohistochemistry |
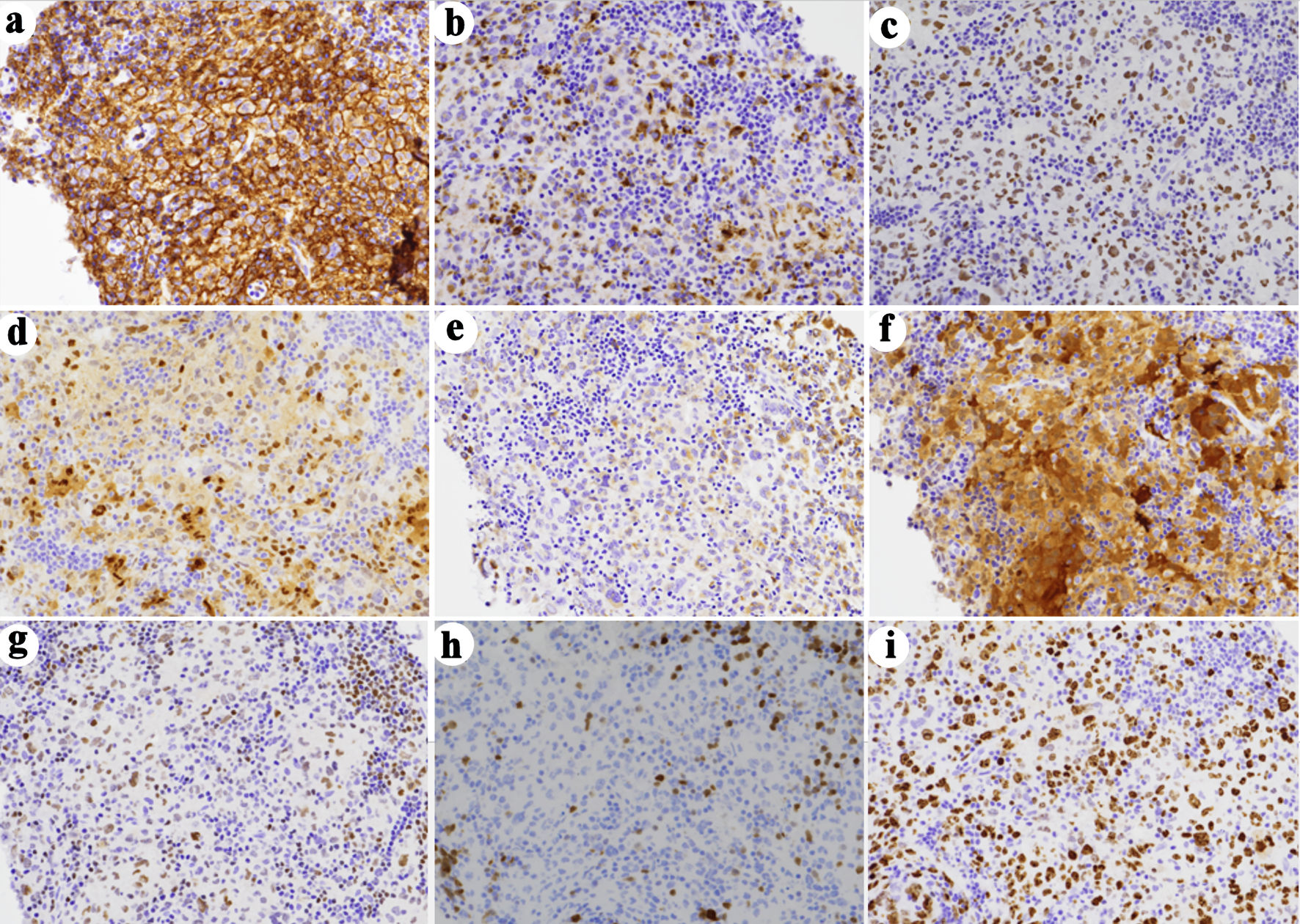 Click for large image | Figure 3. Immunohistochemical (IHC) characterization of the tumor. The histiocytic/dendritic cells are positive for CD4 (a), CD45 (not shown), CD68 (granular and Golgi pattern (b), CD163 (small subset, not shown), BCL6 (c), cyclin D1 (d), lysozyme (e), and S-100 (f), weakly positive for OCT-2 (g) and BCL2 (subset, not shown), and were negative for CD1a (not shown), CD3 (not shown), CD5 (not shown), CD10 (not shown), CD20 (not shown), CD21 (not shown), CD23 (not shown), CD30 (not shown), CD79a (not shown), ALK1 (not shown), BOB.1 (h), MUM1 (not shown), PAX-5 (not shown), and cytokeratin AE1/AE3 (not shown). (i) Ki67 IHC stain shows a high proliferation rate (immunoperoxidase staining; original magnification, × 200). |
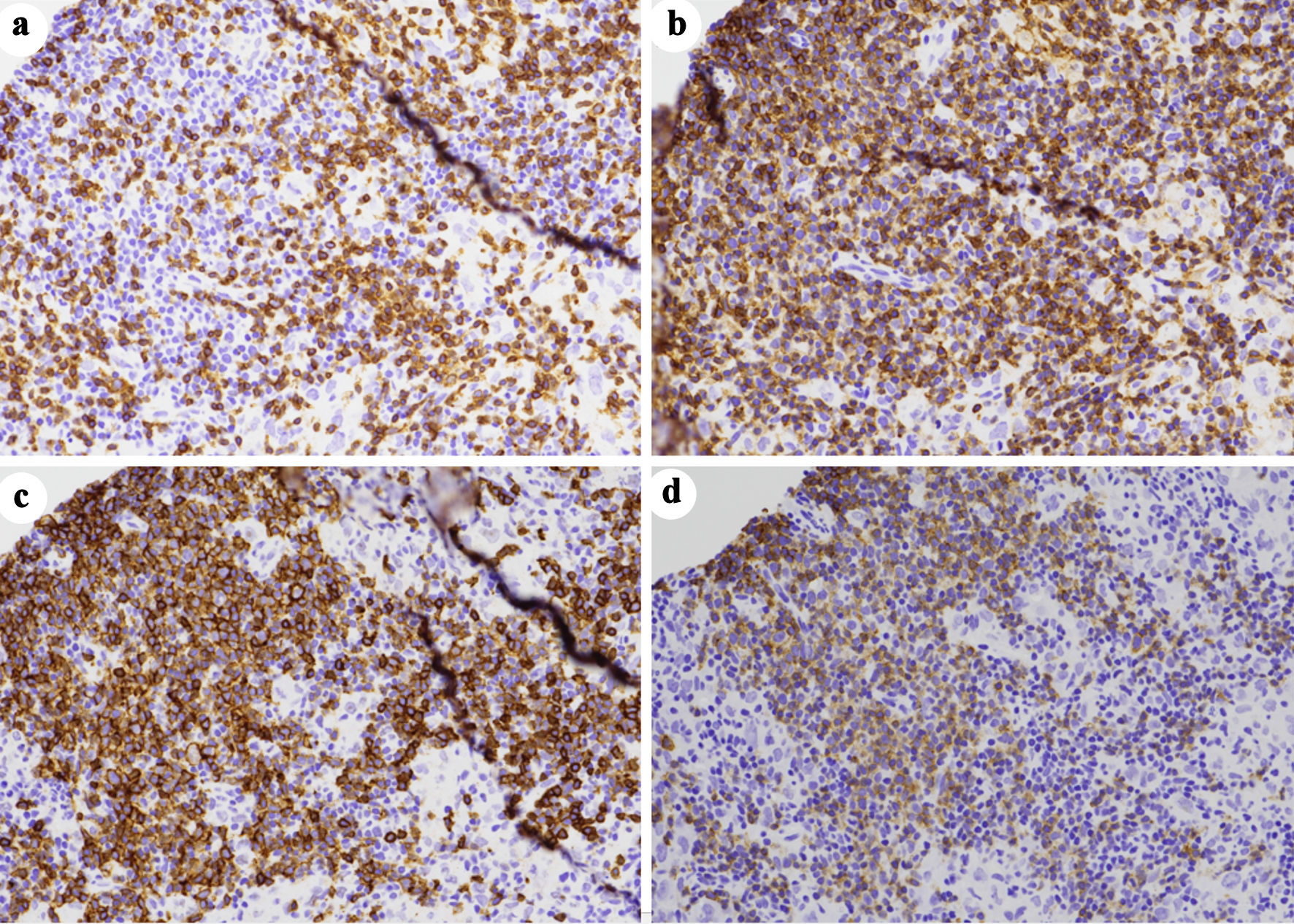 Click for large image | Figure 4. A small focus of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) by immunohistochemistry. The CLL/SLL cells are negative for CD3 (a), but express CD5 (b), CD20 (c), and CD23 (d). The background T-lymphocytes are positive for both CD3 and CD5 (immunoperoxidase staining; original magnification, × 200). CLL/SLL: chronic lymphocytic leukemia/small lymphocytic lymphoma. |
Treatment and follow-up
Given the initial clinical suspicion of Richter transformation, the patient was originally scheduled to begin therapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP). However, after receiving a final diagnosis of histiocytic/dendritic cell sarcoma, the decision was made to initiate bridging therapy with ifosfamide, carboplatin, etoposide (ICE) for three cycles, followed by allogeneic hematopoietic stem cell transplantation (HSCT) instead. Repeat PET/CT was performed, which revealed interval response post-chemotherapy (e.g., SUVmax 9.0 at the nasal-pharyngeal mass, 2.1 - 2.5 at the level IIB cervical nodes, and < 2.5 at the axillary lymph nodes). Unfortunately, approximately 1 month later, the patient had worsening cervical and axillary lymphadenopathy, increased fatigue, and bone pain. The patient underwent repeat PET/CT, which noted an increased SUVmax of 4 at the cervical nodes and 3.5 at the axillary nodes, indicating relapse. A surgical biopsy of five right cervical nodes was performed on one single large lymph node and four smaller lymph nodes. Persistent CLL/SLL was noted in all four smaller lymph nodes. Extensive necrosis was seen in the larger lymph node without any viable histiocytic/dendritic cell neoplasm. The patient was subsequently initiated on rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) in combination with zanubrutinib. She then underwent haploidentical HSCT. At the time of the making of this manuscript, the patient was 45 days post-transplant and had no complaints (Table 3).
 Click to view | Table 3. Treatment Regimens and Responses by Line of Therapy |
| Discussion | ▴Top |
Histiocytic/dendritic cell sarcomas are extremely rare tumors deriving from bone marrow common myeloid progenitor cells that give rise to monocytes, histiocytes, and dendritic cells such as interdigitating dendritic cells and Langerhans cells. They are often associated with an aggressive clinical course [6]. In a small subset of cases, histiocytic/dendritic cell sarcomas arose in the setting of lymphoid malignancy. The histiocytic/dendritic cell sarcomas developed either synchronously or, in most cases, at a later point in time following a preceding lymphoid neoplasm. The most common lymphoid neoplasms associated with histiocytic/dendritic cell sarcomas are follicular lymphoma, CLL/SLL, B-lymphoblastic leukemia/lymphoma, and T-lymphoblastic leukemia/lymphoma [6, 7]. In addition, histiocytic/dendritic cell sarcomas have been reported to be associated with other neoplastic processes such as acute myeloid leukemia (AML), multiple myeloma, mixed germ cell tumors, splenic marginal zone lymphoma, papillary transitional carcinoma of the bladder, and renal cell carcinoma [1, 2, 6]. Patients with concomitant hematologic malignancies (e.g., lymphoma, AML) tend to have a worse prognosis than those without [1].
Several studies have suggested the clonal relationship between histiocytic/dendritic cell sarcomas and associated lymphoid malignancies to postulate that histiocytic/dendritic cell sarcoma can be transdifferentiated from underlying lymphoid neoplasms [2, 6, 8]. The clonal relationship between the neoplastic lymphoid cells and the cells of histiocytic/dendritic lineage was usually determined using laser-capture microdissection technique when the two diseases were mixed. However, in our patient, the lymph node biopsy showed the lymph node was largely replaced by histiocytic/dendritic cell sarcoma in a background of reactive T cells, with only focal minimal CLL/SLL components, whereas her peripheral blood contained CLL cells without evidence of histiocytic/dendritic cell sarcoma. Therefore, we selected the paraffin block of the lymph node biopsy with no obvious CLL/SLL foci for the NGS testing. By NGS, ALK p.T1151M and KRAS p. G13D mutations were detected in both the lymph node and the peripheral blood in our patient, supporting the clonal relationship between the histiocytic/dendritic sarcoma and CLL/SLL. Additional mutations were detected in the lymph node (RBM10 p.K707* and SPEN p.Q3355*) and peripheral blood (TP53 p.Y163C, CCNE1 p.A214V, NF1 p.A706V, and ATM p.L2452R), suggesting clonal evolution. Recurrent karyotypic or molecular abnormalities have not been reported for histiocytic sarcoma or interdigitating dendritic cell sarcoma. Due to the rarity of these entities, it is unknown whether the mutations observed in our case are commonly seen in histiocytic sarcoma or interdigitating dendritic cell sarcoma.
Studies showed that CLL with TP53 disruption, c-MYC abnormalities, unmutated IGHV (< 2%), CD38 gene polymorphisms, stereotypy, non-del(13q), and/or IGHV4-39 gene usage had a relatively high risk for Richter’s transformation [9], while normal cytogenetic findings (four in six cases), deletion of 17p (two in six cases), and/or IGHV4-39 gene usage (four in five cases) were common in the CLL/SLL cells in patients with clonally related histiocytic/dendritic cell sarcoma and CLL/SLL [2]. Chromosomal analysis and CLL FISH panel performed on the peripheral blood of our patient revealed a normal karyotype with negative FISH results for trisomy 12, deletions of 6q, ATM (11q22.3), 13q14, and TP53 (17p13.1). The CLL cells in our patient expressed IgHV3-21 gene family with unmutated IgVH status.
The common clonal origin between CLL/SLL and histiocytic/dendritic cell sarcoma indicates that the histiocytic/dendritic cell sarcoma may represent transformation/transdifferentiation from CLL/SLL. The exact mechanisms of transformation or transdifferentiation are not clear. However, it is possibly due to the direct lineage switch or de-differentiation of neoplastic lymphoid cells to early progenitors followed by redifferentiation of these progenitors to cells of another lineage [4]. Other than the transdifferentiation hypothesis, another possibility is that the neoplastic lymphocytes and the neoplastic histiocytic/dendritic cells may arise from a common precursor that differentiates along different oncogenic pathways [10]. This possibility is less favored given the finding that in no case reported did the histiocytic/dendritic cell tumor precede the diagnosis of the lymphoid neoplasm.
In our patient, the diagnosis of histiocytic/dendritic cell sarcoma was approximately 8 years following the diagnosis of stage 1 CLL. The interval of diagnosis in our patient is within the time frame of prior case reports. This interval has been reported to range from simultaneous diagnosis of CLL/SLL to 11 years after initial diagnosis of CLL/SLL [2, 3]. Our patient received treatment for rheumatoid arthritis including hydroxychloroquine, methotrexate, and six infusions of rituximab before the diagnosis of histiocytic/dendritic cell sarcoma. Although anti-rheumatic drugs, especially methotrexate, can cause lymphoproliferative disease (MTX-LPD), it is unclear if these drugs in this setting affected the transdifferentiation of CLL/SLL to histiocytic/dendritic cell sarcoma in this case. In the study by Feldman et al [8], six of eight patients with clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas were treated prior to the rituximab era, and four of the eight cases were diagnosed prior to any therapy for the primary follicular lymphoma, arguing against the role of rituximab or therapy in altering the lineage of the malignant clone.
Unfortunately, no standard of care or treatment guidelines exist for histiocytic/dendritic cell sarcomas. The treatment modality is customarily based on stage. For localized disease, treatment typically involves upfront surgical resection followed by adjuvant radiation therapy, whereas multi-agent chemotherapy is usually reserved for more extensive disease [11]. Given the rarity of histiocytic sarcoma and interdigitating dendritic cell sarcoma, no single chemotherapy regimen has shown increased efficacy over another. However, due to the aggressive nature of the diseases, similar regimens used to treat diffuse large B-cell lymphoma are commonly used [12]. These regimens include cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or ifosfamide, cisplatin, and etoposide (ICE). For this patient, the initial chemotherapy regimen chosen was ICE, although the rarity of concurrent histiocytic/dendritic cell sarcoma and CLL/SLL and the lack of extensive statistics notably make this occurrence a complex management issue. In one case report, it was documented that interdigitating dendritic cell sarcoma had regressed spontaneously in the setting of SLL without the use of chemotherapy [3]. However, unfortunately, it is also noted that approximately 50% of patients with metastatic interdigitating dendritic cell sarcoma succumb to the disease despite aggressive therapy. The prognostic factors in histiocytic sarcoma and interdigitating dendritic cell sarcoma have yet to be established, but the Eastern Cooperative Oncology Group performance status 2 - 4, Ann Arbor stage III - IV, elevated lactate dehydrogenase (LDH), extra-nodal disease, and bulky or intra-abdominal disease at presentation have been found to be poor prognostic factors in histiocytic or dendritic cell neoplasms [13]. The genetic mutations associated with these neoplasms are also likely to have a prognostic significance. For example, TP53 is considered a gatekeeper for cellular growth and division and, when mutated, can lead to a poor prognosis in numerous malignancies, including non-Hodgkin lymphomas [14]. Genetic alterations in TP53 have been reported to also be associated with early relapse in hematologic malignancies, including acute lymphoblastic leukemia [15]. Although the significance of TP53 mutations in histiocytic/dendritic cell sarcoma has yet to be established, it is possible that the presence of a TP53 mutation in these patients could have led to early relapse and, unfortunately, may confer a poor prognosis in the future.
Learning points
CLL/SLL and other lymphoid neoplasms can transform into histiocytic/dendritic cell sarcoma in extremely rare cases. The diagnosis of this lineage conversion relies on a combination of immunophenotypic analysis and cytogenetic/molecular studies [16]. Although putative mechanisms for transformation have been proposed, additional research is necessary to further elucidate pathogenesis. There are no standard-of-care treatments for concurrent CLL/SLL and histiocytic/dendritic cell sarcoma, and case reporting is critical to help improve patient outcomes.
Acknowledgments
Special acknowledgment to all healthcare professionals involved in the care of this patient.
Financial Disclosure
This work has not received any form of financial support or funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Author Contributions
JC, DFH, GY, AM, and HH did the literature review and drafted the manuscript. JC prepared the figures and figure legends. GY discussed with the patient about the publication and obtained the written consent. JC, DFH, and GY contributed equally, and are co-first authors. AH and XQ edited the manuscript. XQ supervised this work. All authors have read and approved the final manuscript.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
| References | ▴Top |
- Kommalapati A, Tella SH, Durkin M, Go RS, Goyal G. Histiocytic sarcoma: a population-based analysis of incidence, demographic disparities, and long-term outcomes. Blood. 2018;131(2):265-268.
doi pubmed pmc - Shao H, Xi L, Raffeld M, Feldman AL, Ketterling RP, Knudson R, Rodriguez-Canales J, et al. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: a study of seven cases. Mod Pathol. 2011;24(11):1421-1432.
doi pubmed pmc - Khashab T, Sehgal L, Medeiros LJ, Samaniego F. Spontaneous regression of interdigitating dendritic sarcoma in a patient with concurrent small lymphocytic lymphoma. BMJ Case Rep. 2015;2015:bcr2014209014.
doi pubmed pmc - Castro D, Zhang LQ, Nandula S, Choe J, Cai D. Chronic lymphocytic leukemia transdifferentiates into neuroendocrine carcinoma: a case report of a new phenomenon. 2016. North American Journal of Medicine & Science.
- Ghia EM, Jain S, Widhopf GF, 2nd, Rassenti LZ, Keating MJ, Wierda WG, Gribben JG, et al. Use of IGHV3-21 in chronic lymphocytic leukemia is associated with high-risk disease and reflects antigen-driven, post-germinal center leukemogenic selection. Blood. 2008;111(10):5101-5108.
doi pubmed pmc - Egan C, Lack J, Skarshaug S, Pham TA, Abdullaev Z, Xi L, Pack S, et al. The mutational landscape of histiocytic sarcoma associated with lymphoid malignancy. Mod Pathol. 2021;34(2):336-347.
doi pubmed pmc - Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703-1719.
doi pubmed pmc - Feldman AL, Arber DA, Pittaluga S, Martinez A, Burke JS, Raffeld M, Camos M, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111(12):5433-5439.
doi pubmed pmc - Jain P, O'Brien S. Richter's transformation in chronic lymphocytic leukemia. Oncology (Williston Park). 2012;26(12):1146-1152.
pubmed - Fraser CR, Wang W, Gomez M, Zhang T, Mathew S, Furman RR, Knowles DM, et al. Transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma to interdigitating dendritic cell sarcoma: evidence for transdifferentiation of the lymphoma clone. Am J Clin Pathol. 2009;132(6):928-939.
doi pubmed - Susan Joy Philip D, Sherief A, Narayanan G, Nair SG, Av J. Histiocytic sarcoma: clinical features and outcomes of patients treated at a tertiary cancer care center. Cureus. 2022;14(6):e25814.
doi pubmed pmc - Oto M, Maeda A, Nose T, Ueda Y, Uneda S, Inadome A, Oshima K, et al. Retroperitoneal bulky histiocytic sarcoma successfully treated with induction chemotherapy followed by curative surgery. Intern Med. 2017;56(20):2765-2768.
doi pubmed pmc - Shimono J, Miyoshi H, Arakawa F, Sato K, Furuta T, Muto R, Yanagida E, et al. Prognostic factors for histiocytic and dendritic cell neoplasms. Oncotarget. 2017;8(58):98723-98732.
doi pubmed pmc - Xu P, Liu X, Ouyang J, Chen B. TP53 mutation predicts the poor prognosis of non-Hodgkin lymphomas: Evidence from a meta-analysis. PLoS One. 2017;12(4):e0174809.
doi pubmed pmc - Salmoiraghi S, Montalvo ML, Ubiali G, Tosi M, Peruta B, Zanghi P, Oldani E, et al. Mutations of TP53 gene in adult acute lymphoblastic leukemia at diagnosis do not affect the achievement of hematologic response but correlate with early relapse and very poor survival. Haematologica. 2016;101(6):e245-248.
doi pubmed pmc - Stoecker MM, Wang E. Histiocytic/dendritic cell transformation of B-cell neoplasms: pathologic evidence of lineage conversion in differentiated hematolymphoid malignancies. Arch Pathol Lab Med. 2013;137(6):865-870.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


