| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 4, Number 1, March 2015, pages 125-130
Attached Segments and Cryovial Samples as a Useful Tool in Cord Blood Banking Quality Control
Sophia Andriopouloua, Ioannis Anagnostakisb, Efstathios Michalopoulosa, c, Effrosyni Panagoulia, Theofanis Chatzistamatioua, Andreas Papassavasa, Catherine Stavropoulos-Giokasa
aHellenic Cord Blood Bank (HCBB), Biomedical Research Foundation, Academy of Athens, Greece
b401 General Military Hospital, Athens, Greece
cCorresponding Author: Efstathios Michalopoulos, Hellenic Cord Blood Bank (HCBB), Foundation for Biomedical Research, Academy of Athens, 4 Sorranou Efessiou Street, 11527 Athens, Greece
Manuscript accepted for publication March 18, 2015
Short title: Comparison of CB Segments and Cryovials
doi: http://dx.doi.org/10.14740/jh198w
| Abstract | ▴Top |
Background: Assessment of the umbilical cord blood (UCB) unit is a crucial issue in the hematopoietic stem cell transplantation. A cord blood bank (CBB) must ensure that the final product of transplantation is represented accurately from quality control (QC) products of the UCB unit. The use of a tube segment attached to the UCB unit as well as a separate 1.8 mL cryovial containing an identical aliquot of the cryopreserved UCB unit product was exposed to the same post-processing freezing and storage conditions as the UCB unit. Eventually, the utility of the attached segment and the 1.8 mL cryovial was evaluated as valid QC tools in order the final UCB unit graft to be validated.
Methods: A total of 30 cord blood (CB) units, stored in liquid nitrogen, with their attached segments and 1.8 mL cryovials were thawed and several QC parameters (total nucleated cells (TNCs), CD34+ cells, CD133+ cells, percent viability and recovery) were obtained. Functional tests such as clonogenic assays were also performed.
Results: Non-statistical differences were observed between UCB units, attached segments and 1.8 mL cryovials for any of the examined parameters. The expected clonogenic efficiency (ECLONE) was above 80% for all the three kinds of thawed samples (UCB unit, attached segment and QC sample).
Conclusions: The QC of attached segment and 1.8 mL cryovial linked to the cryopreserved UCB unit may be used as a means to predict the potency and functionality of the actual UCB unit before transplantation.
Keywords: Attached segments; Cryovial samples; Cord blood banking; Quality control
| Introduction | ▴Top |
UCB has been an increasingly utilized source of hematopoietic stem cells (HSCs) for transplantation of patients with advanced and high-risk hematological diseases [1]. Among the benefits of CB, compared to bone marrow (BM) and mobilized peripheral blood (MPB), as a source of engrafting HSCs and hematopoietic progenitor cells (HPCs) are its ready availability through CBBs, and a relatively low level of graft vs. host disease (GVHD) elicited after transplantation. To date more than 20,000 CB transplants have been performed for HSC reconstitution after myeloablative therapy in malignant and non-malignant diseases [2].
Since the first unrelated cord blood banking program, which started at the New York Blood Center in 1991 [3], a number of public cord blood banking programs have been established throughout the world in order to cryogenically store CB units for potential transplantation to unrelated and related recipients [4].
Clinical experience has highlighted two chief benefits of CB. Firstly, as a cryopreserved HSC source, CB is rapidly available with an absence of donor attrition and has the advantage that a transplant can be scheduled according to patient needs rather than donor availability. Secondly, in comparison to adult HSC graft, the incidence of GVHD is lower in CB transplants without the loss of a graft-versus-malignancy effect. However, the quality of a CB graft might be affected during the freezing or thawing process and transportation to the transplant unit. Therefore, optimal CB unit processing, cryopreservation, and storage are critical to ensure the safety and potency of the graft [5].
Although selection of a CB unit by a transplant center mainly relies on its degree of donor-recipient human leukocyte antigen (HLA) compatibility and its TNC content, the predictive value of other CB characteristics on transplant outcome has been reported [6]. These include CD34+ cell content [6], hematopoietic colony-forming cell (CFC) content [7], and nucleated red blood cell (nRBC) content. All of these variables are normally defined before cryopreservation [6].
The purpose of this study was to examine and evaluate the utility of an integral segment and the 1.8 mL cryovial containing aliquots of cryopreserved product that have been exposed to the same cryostorage conditions as the CB unit, as QC tools for CB banking. Furthermore, critical QC variables were assessed, such as TNCs, CD34+ cells among others that, more or less, safely predict hematopoietic potential and viability of cryopreserved units [7-9].
| Materials and Methods | ▴Top |
CB collection
Thirty CB units were collected, by trained maternity clinic personnel after informed consent. CB was collected from the umbilical vein by gravity drainage (either with in utero or ex utero technique) in a sterile disposable single CB collection (MSC 1201 DU, MacoPharma, Tourcoing, France) that also contained 29 mL of CPD. After labeling, the UCB units were stored at 4 - 8 °C and distributed to the Hellenic Cord Blood Bank of Biomedical Research Foundation of Academy of Athens. Processing of all UCB units was performed within 48 h of collection. The UCB units that were used in this study have been rejected as not suitable for transplantation mainly for low TNC, and consequently, low CD34+ cell numbers and the length of storage of the UCB units ranged from 3 to 12 months.
Volume reduction
After removal of samples for pre-freeze QC testing, hydroxyethyl starch (HETASTARCH-6% HES in 0.9 NaCl (130 kDa); Cooper SA) was added to the CB collection bag at a final concentration of 20% (v/v). Once diluted, the UCB unit was centrifuged at 50 g for 5 min at 10 °C. This step was followed by removing the supernatant (which contains plasma and nucleated cells) with the use of a plasma extractor (Auto-Volume Expressor Operator Manual, Thermogenesis), the RBC bag was sealed off and the remaining bags (plasma and buffy coat) were centrifuged at 400 g for 10 min at 10 °C. Finally, the supernatant was removed with the plasma extractor, the bag containing plasma was removed and the remaining freezing bag, which contained the buffy coat with the majority of the HSCs, was stored at 4 - 8 °C till the start of cryopreservation process.
Cryopreservation
A 50% solution of dimethyl sulfoxide, diluted in dextran 40 (791-04U, Pall, East Hills, NY) was used at a final concentration of 10% (v/v) to the final product. At this point, 0.5 mL of the final product was transferred to a 1.8 mL cryovial (QC sample). Also, three segments were sealed on the attached tube, with 200 μL approximate volume. All the products were then cryopreserved with the use of a controlled-rate freezer (SY-LAB Rate Freezer Instruments, Neupurkersdorf, Austria). After freezing at -100 °C, the bag with the segments and the QC sample were deposited in a liquid nitrogen tank (< -186 °C).
Thawing and washing
On removal from the liquid nitrogen tank, after stored from 3 to 12 months, the CB freezing bags, accompanied with the related segments and the QC samples, were exposed to ambient air for 5 min and then immersed in a 37 °C water bath for rapid thawing (2 - 3 min). A thawing kit adapted for the freezing bag was used (stem cell transplant system; Pall Medsep), and then the CB was diluted with an equal volume of an isotonic salt solution containing 2.5% human albumin (human-albumin 20%, Aventis-Behring, Marburg, Germany) and 6% HES with continuous mechanical mixing. After 5 min of agitation, the UCB unit was diluted, adding 60 mL of 6% HES. The UCB unit was then centrifuged at 450 g for 20 min at 10 °C, the supernatant was removed with the aid of the plasma extractor, and the sedimented cells were resuspended in fresh albumin to the volume originally collected after the volume reduction. Contents of the segments were removed with a tuberculin syringe (1 cm3, 28.5 gauge, Becton Dickinson).
Hematologic cell counts
For every sample, a full blood differential cell count was performed using a hematology analyzer (model MEK-6318K, Nihon Kohden, Rosbach, Germany) and absolute cell counts and corresponding recoveries for each cell category (TNCs, mononuclear cells (MNCs), nRBCs, and platelets) were calculated.
Flow cytometry
The samples were dual labeled after incubation with anti-CD45+ (fluorescein isothiocyanate) and anti-CD34+ (phycoerythrin)-conjugated antibodies. Furthermore, the viability of CD34+ cells was assessed by using 7-aminoactinomycin D. For the flow cytometric analysis, a flow cytometer with the accompanying software (Beckman-Coulter Epics XL Flow Cytometer and Analysis Section with CD34 Analysis Template-PROCOUNT, Beckman Coulter, Nyon, Switzerland) was used with the reagents from Beckman Coulter (Stem-Kit, Ref. IM3660). A dual-platform protocol was used for the determination of CD34+ cells and CD45+ cells number according to ISHAGE (International Society for Hematology and Graft Engineering) guidelines for gating.
In vitro colony assays to determine colony-forming units (CFUs)
For the determination of CFUs, a commercial semisolid methylcellulose medium (Methocult GF H4434, StemCell Technologies, Vancouver, BC, Canada) was used. Briefly, MNCs were plated in duplicate at concentrations of 1.0 × 104 to 4.0 × 104 nucleated cells/mL per petri dish. One drop (approximately 4 μL) of the thawed cell suspension was added to 3 mL of methylcellulose media and cultured as described below.
After 14 - 16 days of incubation at 37 °C in humidified air and 5% CO2, granulopoietic clonogenic progenitors of granulocytes macrophages (CFU-GM), primitive erythroid progenitors burst-forming units, erythroid (BFU-E), CFUs-erythroid (CFU-E), and multipotential colonies (CFU-GEMM) were scored for growth by microscopic examination based on standard protocols (StemCell Technologies) using an inverted microscope (model DFK 72AUCO2, The Imaging Source, Stuttgart, Germany).
Statistical analysis
Computer software (Minitab, Release 13.1, Minitab, Inc., State College, PA) was used for statistical analysis. Linear correlation and the two-tailed paired t tests were used to compare certain CB variables pre-freeze and post-thaw. Results are presented as mean ± 1 standard deviation (SD) and were considered significant at P values of not more than 0.05.
| Results | ▴Top |
Attached segment and QC sample cell content (TNCs, CD34+) represent accurately the corresponding UCB unit content
Thirty (30) UCB units, stored frozen in liquid nitrogen containers at temperature < -186 °C, along with the corresponding attached segments and QC samples were thawed and evaluated.
No statistically significant difference was observed between cell counts (TNCs, CD34+ cells) of the attached segment, QC sample, and UCB unit with the mean of TNC absolute numbers to drop from 768.40 ± 90.57 × 106 cells (range: 613 - 926) for fresh UCB unit to 636.4 ± 94.42 × 106 (range: 483 - 808) after thawing. The TNC number for the attached segment was 595.57 ± 80.54 × 106 (range: 451 - 710) and for the QC sample was 597.5 ± 91.41 × 106 (range: 450 - 783). TNC percent viability remained relatively stable among UCB unit, attached segments and QC samples after thawing with the inevitable deterioration not to be significant (90.57 ± 1.98 × 106, range: 87-94) for the UCB bag, post-thaw. As for the attached segment and QC sample obtained viability, no statistical differences were found between them and the corresponding UCB unit (P = 0.09 and 0.07 respectively) and correlated strongly with relative UCB viability (Table 1).
 Click to view | Table 1. UCB Content and TNC Percent of Viability Before Cryopreservation and After Thawing and Its Correlation With Corresponding Values of Attached Segment and QC Sample |
For the fresh UCB unit the mean number of CD34+ cells was 1.02 ± 0.33 (range: 0.41 - 1.53) and for after thawing 0.93 ± 0.33 (range: 0.35 - 1.45). Furthermore, the mean number of CD34+ cells for the attached segment was 0.89 ± 0.29 (range: 0.40 - 1.46) and for QC sample 0.90 ± 0.33 (range: 0.33 - 1.43) (Table 1). No statistical difference was found in CD34+ cells between QC samples, attached segments and the UCB unit (P = 0.62 and 0.75 respectively).
Comparison of CFUs between UCB unit, attached segment and QC sample
The total mean CFU value after thawing was 82.69 ± 24 × 104 (range: 36.49 - 128.13) colonies for the UCB unit, 74.52 ± 18.08 × 104 (range: 37.10 - 106.57) for the attached segment (P = 0.14) and 75.47 ± 19.81 × 104 (range: 37.68 - 101.32) for the QC sample (P = 0.21). Both segment and QC sample showed strong linear correlation with the defrosted UCB unit (Table 1).
The percent total recovery of TNCs and CD34+ cells (for the UCB unit) after thawing was satisfactory: 82.62 ± 4.42 (range: 69.1 - 89.1) for TNCs and 89.75 ± 5.27 (range: 76.1 - 97.7) for CD34+ (Table 2). The difference in recovery between TNCs and CD34+ along with the similar difference in viability was expected because TNC population includes mature cells, e.g. PMNs that are far more sensitive to ultralow temperatures and freezing-thawing procedures. Also, the differences between percent total recovery of CD34+ cells and TNCs after thawing, for the attached segment and the QC sample, and UCB unit, when compared, were not statistically significant. The same occurred with percent CFU total recovery with the differences among the calculated recoveries in all categories of samples (UCB units, attached segments and QC samples) not to be statistically significant (Table 2).
 Click to view | Table 2. Percentage of CFU and Cell (TNC and CD34+) Recovery After Thawing for UCB, Attached Segment and QC Sample |
It is interesting to point out that the calculated recoveries along with cell numbers (TNC, CD34+ cells) and CFUs of the attached segment and the QC sample correlated strongly with the corresponding values of the thawed UCB unit (Table 1, 2). Figures 1 and 2 show the magnitude of the correlation between post-thaw CD34+ of UCB units and the corresponding values from QC sample and attached segment.
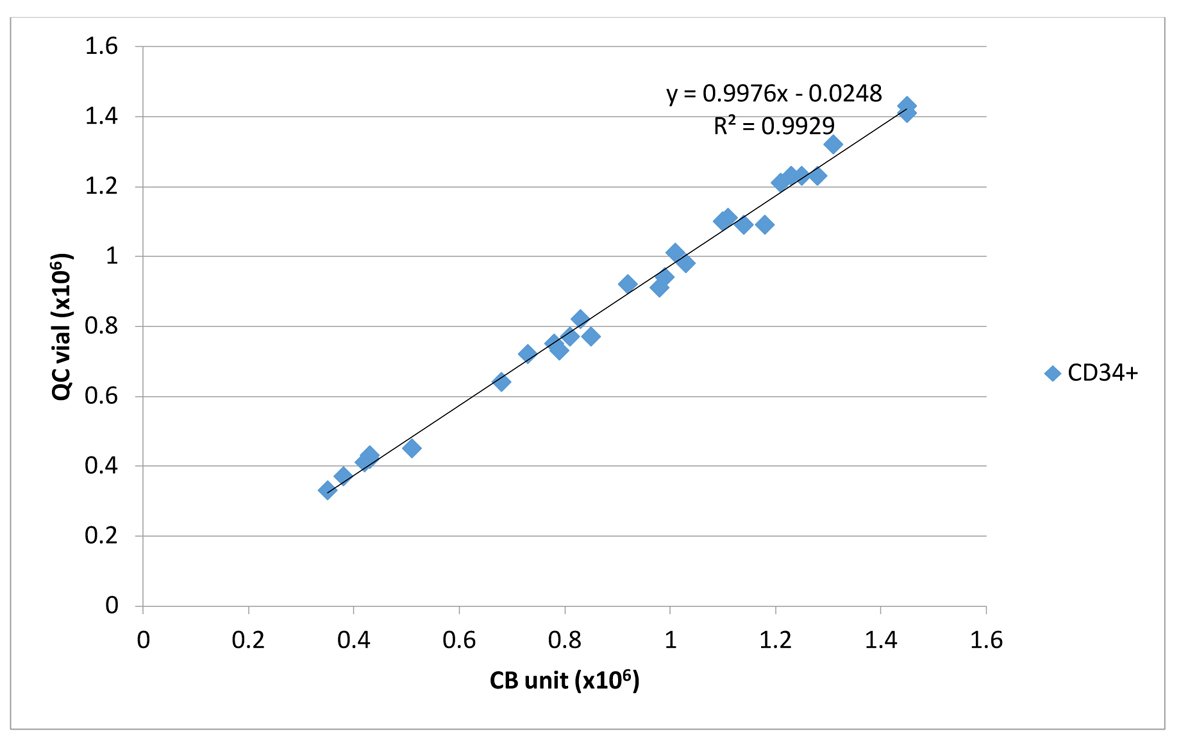 Click for large image | Figure 1. Linear correlation between CD34+/UCB unit and CD34+/QC cryovial. |
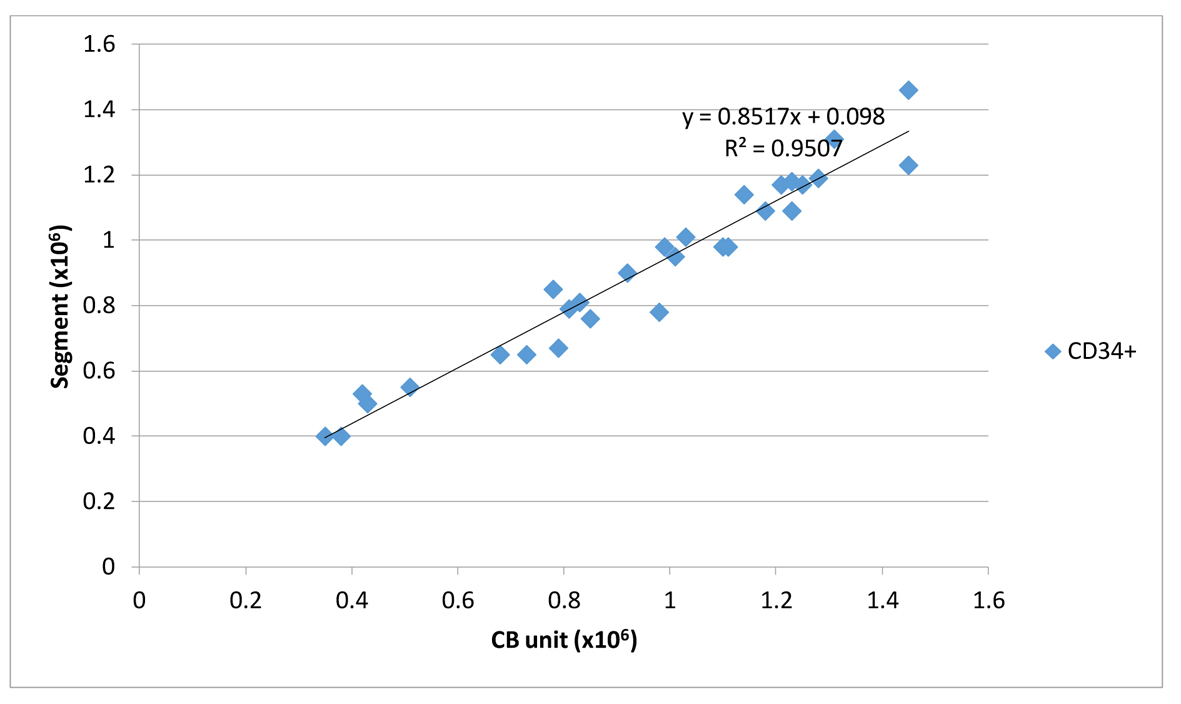 Click for large image | Figure 2. Linear correlation between CD34+/UCB unit and CD34+/attached segment. |
Finally, the expected clonogenic efficiency (ECLONE), parameter that could be used for qualitative assessment of graft potency, was above 80% for all the three kinds of thawed samples (UCB unit, attached segment and QC sample) and correlated well with R2 0.87 between segment/thawed UCB unit and R2 0.88 between QC sample/thawed UCB unit (Table 1).
| Discussion | ▴Top |
UCB is a great source of HPCs for allogeneic transplantation, especially [10] for patients without suitably matched and readily available related or unrelated stem cell donors [5], for the treatment of a wide variety of malignant and non-malignant disorders [2].
UCB units are stored, usually in liquid nitrogen tanks, for long time periods (months to years) in CBBs. The suitability of CB stem and progenitor cell components prepared for transplantation is evaluated primarily by determination of variables such as TNCs [11, 12], CFUs [13], and CD34+ cell numbers [14] which are obtained before cryopreservation [15] without taking into account that the product may lose potency during liquid nitrogen storage, be exposed to temperatures outside the recommended storage range, or may be accidentally thawed [9].
Moreover, the election of a UCB unit for transplantation has some degree of uncertainty due to the different protocols, lack of standardized techniques, CBBs, and transplant centers involved in this process [7], so there is also a developing interest in assessing quality characteristics after thawing, which may be more indicative of the actual functional cell number [15]. Additionally, over time laboratory practices and personnel evolve. Therefore, the application, as a standard procedure for all UCB units stored in the CBB, of a tool such as the analysis of a contiguous segment that provides critical information about the overall quality of the UCB unit is necessary [9].
In this study, we utilized an integral bag segment and a cryovial, both containing aliquots of cryopreserved product, in order to assess critical QC variables of a given UCB unit as a means to evaluate the potency of the contained HPCs and to predict transplantation success [16].
The use of a tube segment attached to the UCB unit containing an identical aliquot of the cryopreserved product that is exposed to the same storage conditions as the unit has been proposed as a QC tool for UCB banking by some investigators [8, 9]. However, this requires that the CBB standardizes and validates a reproducible method to generate and analyze these samples [7].
Cell counts, CD34 analysis, and CFU assessment are recommended by UCB standards for the qualification of the graft, and all of them can be performed from the segment attached to the UCB unit. Taken together, our results suggest that, for all the variables assessed (TNC, CD45, CD34, and CFU), the number of cells and viability in the attached segment with this particular freezing bag and with the freezing protocol described will be representative of the graft [7].
The comparison between thawed bag, segment and cryovial samples [16], showed that all variables under investigation (TNC, CD34+ counts, viability, and clonogenic assays) were strongly correlated, fact that indicates that CB from segments and/or cryovials are indeed representative of CB in main bags [17].
It is interesting to note that the CFU/CD34+ ratio (ECLONE) determined in the UCB unit was conserved in the attached segment and the corresponding QC sample.
These findings are supported by the fact that the CFU/CD34 ratio has been already reported as a good predictor in short-term platelet engraftment in mobilized blood progenitor cell transplantation [7, 18]. The CFU-GM/CD34+ ratio (ECLONE), the percentage of colonies generated per CD34+ cell seeded, is proposed by some investigators to be an apparent predictor of earlier platelet engraftment, suggesting that the ratio reflects the engraftment potential of donor progenitor cells [18].
In the case of a successful cryopreservation, that is without severe degradation of the product that would possibly compromise the success of a possible CB transplantation, the percentage of colonies generated per CD34+ cell seeded is expected to remain fairly constant in a specific unit after thawing (ECLONE). By assessing the specific ECLONE of a progenitor source, we could define normality ranges for the qualitative detection of functional defects after cryopreservation by using the colony score.
Of course there are limitations with the use of the integral segments and cryovials. For instance, the product can only be tested once or twice prior to use since there is a relatively small number of segments or cryovials connected to a given UCB unit with adequate HPCs number for confirmatory HLA testing, but not for extensive viability testing. Additionally, the CFU assay requires 14 days, a fact that further limits the validity of the assay because sometimes the UCB unit is needed for transplant prior to completion of the CFU assay. However, given that the length of preparative regimens is usually 7 days, in most instances the CFU results would be available prior to infusion. Lastly, even though strong correlation has been found between CFU results from segment/cryovial and the actual defrosted UCB unit, caution should be used in their interpretation, since there is considerable variability with CFU as an assay for hematopoiesis. The presence of CFU in the segment or the cryovial supports the fact that the unit contains viable hematopoietic progenitors within the product intended for transplantation, although the clinical utility is possibly limited to confirming the presence of hematopoietic precursors within the product, and not as a quantitative measure of hematopoietic potential [9].
Finally, at the transplant center, the attached segment with the addition of a cryovial may serve as a means to assess the progenitor cell content of the unit according to its own analysis techniques and stringencies without compromising the CB graft [7] and that such analyses could be a useful tool as a QC and the determination and confirmation of the product viability [7, 9].
| References | ▴Top |
- van Burik JA, Brunstein CG. Infectious complications following unrelated cord blood transplantation. Vox Sang. 2007;92(4):289-296.
pubmed - Broxmeyer HE: Cord blood hematopoietic stem cell transplantation. In: StemBook. Cambridge (MA), 2008.
- Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92(22):10119-10122.
doi pubmed - Lubin BH, Shearer WT. Cord blood banking for potential future transplantation. Pediatrics. 2007;119(1):165-170.
doi pubmed - Ballen KK, Barker JN, Stewart SK, Greene MF, Lane TA. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. 2008;14(3):356-363.
doi pubmed - Wagner E, Duval M, Dalle JH, Morin H, Bizier S, Champagne J, Champagne MA. Assessment of cord blood unit characteristics on the day of transplant: comparison with data issued by cord blood banks. Transfusion. 2006;46(7):1190-1198.
doi pubmed - Rodriguez L, Garcia J, Querol S. Predictive utility of the attached segment in the quality control of a cord blood graft. Biol Blood Marrow Transplant. 2005;11(4):247-251.
doi pubmed - McKenna D, McCullough J. Clinical utility of the clonogenic assay in the QC of UCB units for transplant [abstract 167]. 10th Annual ISCT Meeting, Dublin, 2004:71.
- Goodwin HS, Grunzinger LM, Regan DM, McCormick KA, Johnson CE, Oliver DA, Mueckl KA, et al. Long term cryostorage of UC blood units: ability of the integral segment to confirm both identity and hematopoietic potential. Cytotherapy. 2003;5(1):80-86.
doi pubmed - Cohen Y, Nagler A. Umbilical cord blood transplantation--how, when and for whom? Blood Rev. 2004;18(3):167-179.
doi - Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28(11):1197-1205.
doi - Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565-1577.
doi pubmed - Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96(8):2717-2722.
pubmed - Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611-1618.
pubmed - Kurtz J, Seetharaman S, Greco N, Moroff G. Assessment of cord blood hematopoietic cell parameters before and after cryopreservation. Transfusion. 2007;47(9):1578-1587.
doi pubmed - Solves P, Planelles D, Mirabet V, Blasco I, Carbonell-Uberos F, Soler MA, Roig RJ. Utility of bag segment and cryovial samples for quality control and confirmatory HLA typing in umbilical cord blood banking. Clin Lab Haematol. 2004;26(6):413-418.
doi pubmed - Lee HR, Shin S, Yoon JH, Roh EY, Song EY, Han KS, Kim BJ. Attached Segment Has Higher CD34(+) Cells and CFU-GM Than the Main Bag After Thawing. Cell Transplant. 2015;24(2):305-310.
doi pubmed - Fu SQ, Abboud CN, Brennan JK, Ifthikharuddin JJ, Nichols D, Liesveld JL. Impact of mobilized blood progenitor cell quality determined by the CFU-GM/CD34+ ratio on rapid engraftment after blood stem cell transplantation. Blood Cells Mol Dis. 2002;28(3):315-321.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


