| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 4, Number 2, June 2015, pages 171-173
Coombs-Negative Autoimmune Hemolytic Anemia Associated With Liver Cirrhosis Due to Hepatitis C Virus
Mikiko Okazakia, Takakazu Higuchib, e, Ryosuke Koyamadab, Sadamu Okadab, Yoshiyuki Fujitac, Toyomi Kamesakid
aDepartment of Internal Medicine, St. Luke’s International Hospital, Tokyo, Japan
bDivision of Hematology, St. Luke’s International Hospital, Tokyo, Japan
cDivision of Gastroenterology, St. Luke’s International Hospital, Tokyo, Japan
dCenter for Community Medicine, Jichi Medical University, Tochigi, Japan
eCorresponding Author: Takakazu Higuchi, St. Luke’s International Hospital, 1-9 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan
Manuscript accepted for publication April 23, 2015
Short title: Coombs-Negative AIHA in Liver Cirrhosis
doi: http://dx.doi.org/10.14740/jh202w
| Abstract | ▴Top |
An 83-year-old woman who had been treated for liver cirrhosis due to hepatitis C virus (HCV) had progressive thrombocytopenia and anemia. Coombs-negative autoimmune hemolytic anemia (AIHA) was diagnosed based on the findings of hemolysis and the increased number of the red blood cell-bound immunoglobulin G molecules. The increased level of the platelet-associated immunoglobulin G and refractoriness to platelet transfusions suggested involvement of autoimmune mechanism in the thrombocytopenia. Oral prednisolone was effective for AIHA and thrombocytopenia. Global derangement of the immune system associated with HCV was suggested. As increased serum bilirubin and lactate dehydrogenase levels are common laboratory findings in patients with chronic HCV liver diseases, it is likely that a considerable number of patients complicated with Coombs-negative AIHA, which is a medically treatable anemia, are left without being noticed.
Keywords: Hepatitis C virus; Liver cirrhosis; Autoimmune hemolytic anemia; Coombs test
| Introduction | ▴Top |
Hepatitis C virus (HCV) infection not only causes chronic liver diseases but is frequently associated with extrahepatic autoimmune manifestations [1-4]. The clinical significance of such autoimmune phenomena is highly diverse, ranging from subclinical or laboratory abnormalities to overt clinical manifestations that can be severe in some patients. Immune thrombocytopenia is reported to be complicated in a considerable number of patients with chronic HCV infection [5-10]; however, the frequency and the significance of the complication of autoimmune hemolytic anemia (AIHA) have never been extensively studied [11].
We herein report a case of liver cirrhosis due to chronic HCV infection complicated with Coombs-negative AIHA, which is a medically treatable anemia but may be left unrecognized in patients with HCV chronic liver diseases.
| Case Report | ▴Top |
An 83-year-old woman had been treated at St. Luke’s International Hospital and her family physician for liver cirrhosis due to chronic HCV infection possibly infected when she had a blood transfusion at delivery 54 years earlier. She had not received interferon therapy or any other antiviral agents and been treated with ursodeoxycholic acid. Hepatcellular carcinoma (HCC) had been detected 4 years earlier and treated with transcatheter arterial embolization and radiofrequency ablations. She had been diagnosed as essential tremor, hypothyroidism, and type 2 diabetes and taking medications for these complications. There was no family history of autoimmune diseases. She had a blood test 2 weeks before admission and the platelet count was 119 × 109/L. Several days later, she noticed petechiae and purpurae on the extremities which rapidly extended to the trunk and was admitted.
On admission, generalized subcutaneous and oral mucosal hemorrhages were noted. The liver and the spleen were not palpable. The white blood cell count was 3.9 × 109/L, the red blood cell (RBC) count was 2.87 × 1012/L with 6.1% of reticulocytes, the hemoglobin (Hb) level was 10.9 g/dL, and the hematocrit level was 31.2%. The platelets count was 6.0 × 109/L. The blood chemistry showed that the total protein level was 9.1 g/dL, the total bilirubin level was 2.5 mg/dL, the direct bilirubin level was 1.8 mg/dL, the aspartate transaminase level was 84 U/L, the alanine transaminase level was 55 U/L, and the lactate dehydrogenase (LDH) level was 301 U/L (reference range: 118 - 223). The serum ferritin level was 930 ng/mL and the haptoglobin level was below the limit of detection. The electrophoresis of the serum protein revealed polyclonal increase of the γ-globulin and the serum immunoglobulin (Ig) G level was 4,768 mg/dL. Antinuclear (homogenous type), anti-thyroglobulin, and anti-thyroid peroxidase antibodies were positive. The quantitative RT-PCR detected 6.8 log IU/mL HCV RNA. Conventional direct and indirect Coombs’ tests were both negative. The bone marrow aspiration study revealed a hypocellular marrow without apparent dysplasia. Radiographic studies did not reveal any signs of HCC.
Platelet concentrates were transfused; however, the platelet count hardly responded to the transfusions and there was a rapid decline in the Hb level (Fig. 1). The platelet-associated immunoglobulin G (PAIgG) level was elevated to 457.5 ng/107 cells (reference range: 5.0 - 25.0). Moreover, a quantitative assay of anti-RBC antibodies revealed that 142 IgG molecules were bound to one RBC, which is above the cutoff value for the diagnosis of Coombs-negative AIHA [12]. Based on these findings, autoimmune destruction of RBCs was considered to be the major mechanism of the anemia rapidly progressed in a patient with preexisting cytopenias associated with liver cirrhosis. Autoimmune mechanism was also considered to be involved in the thrombocytopenia. Oral prednisolone (PSL) therapy was initiated at a dose of 30 mg/day. The anemia and thrombocytopenia responded promptly with steady increases in both RBCs and platelets. The dose of PSL was gradually tapered and she was discharged on the 70th hospital day. The RBCs and platelets were maintained for about 1 year with PSL at 5 mg/day until the death due to the relapse of HCC.
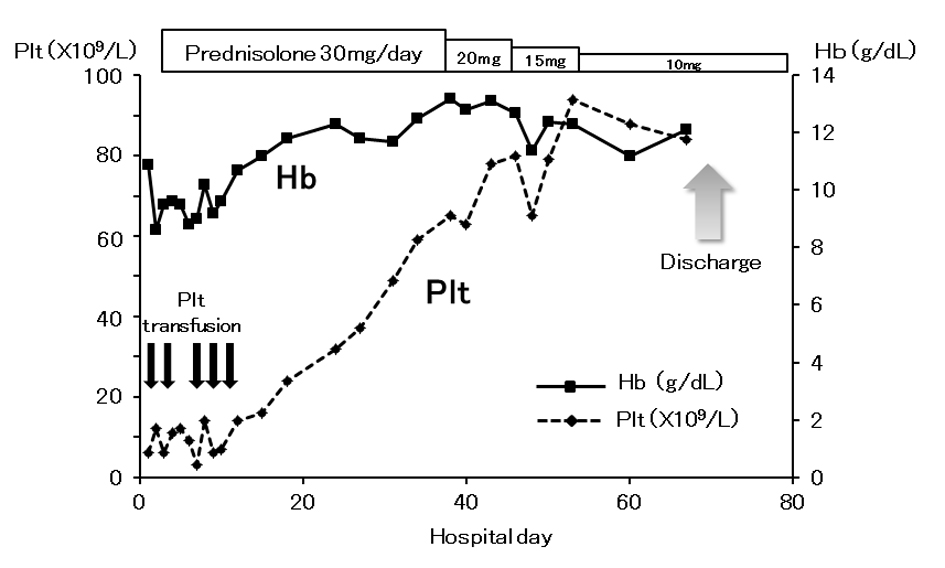 Click for large image | Figure 1. Clinical course during admission. Changes in the hemoglobin level (Hb, straight line) and the platelet count (Plt, dotted line) and the treatment given are illustrated. |
| Discussion | ▴Top |
HCV infection is associated with development of a variety of both non-organ specific and organ specific autoantibodies [1-4]. Diverse mechanisms leading to extrahepatic autoimmune manifestations in chronic HCV infection are proposed, including direct infection of HCV on B lymphocytes leading to their expansion, molecular mimicry between HCV protein and self-antigens, reduction in the T regulatory cell number, and activation of B lymphocytes through the binding of HCV envelop protein 2 and CD81 on the surface of B lymphocytes and the increase of B lymphocyte activating factor [2-4].
On the other hand, it is well recognized that a variety of cytopenias of various degrees are frequently complicated with chronic liver diseases [13]. Thrombocytopenia is the most frequently observed hematological complication and seen in up to 76% of patients [14, 15]. Multiple factors are assumed to contribute to the development of thrombocytopenia, including splenic sequestration, suppression of the production in the bone marrow, and decreased thrombopoietin level [13-15]. Moreover, autoimmune mechanisms are considered to play a role in the pathogenesis of HCV-associated thrombocytopenia. In the present case, involvement of autoimmune mechanism was suggested by the elevated PAIgG level, refractoriness to platelet transfusions, and prompt response to corticosteroid.
Anemia is another common complication of chronic liver diseases [13, 16]. The causes of anemia include acute or chronic gastrointestinal hemorrhage and hypersplenism secondary to portal hypertension. In addition, alterations in the composition of the lipids in the RBC membrane due to the derangement in the lipoprotein metabolism may lead to hemolysis and, in cases of chronic HCV infection, antiviral therapies with ribavirin may cause hemolytic anemia [13, 16]. However, the association between AIHA and chronic HCV infection has not been explored except a cohort study [8] and a case series [11] and the frequency and significance of anti-RBC antibodies causative of Coombs-negative AIHA among patients with chronic HCV liver diseases have never been studied. Considering the high frequency of various autoantibodies among patients with chronic HCV infection, we assume that the frequency of anti-RBC antibodies is substantially high among patients with chronic HCV liver diseases. As the laboratory findings of hemolysis, namely elevated indirect bilirubin and LDH levels, are also common laboratory findings observed in patients with chronic liver diseases, it is highly possible that patients with mild to moderate hemolysis are left unrecognized. Therefore, we believe that autoimmune hemolysis contributes to the development of anemia in a considerable number of patients with chronic HCV liver disease and that the quantitative assay of anti-RBC antibodies should be performed in patients with chronic HCV liver disease and anemia, especially when other autoantibodies are detected as global derangement of the immune system associated with HCV may be present in such patients. Awareness of this complication and large studies are definitely needed to elucidate the contribution of this medically treatable anemia to the etiology of anemia seen in patients with chronic HCV liver diseases.
| References | ▴Top |
- Pivetti S, Novarino A, Merico F, Bertero MT, Brunetto MR, Bonino F, Caligaris-Cappio F. High prevalence of autoimmune phenomena in hepatitis C virus antibody positive patients with lymphoproliferative and connective tissue disorders. Br J Haematol. 1996;95(1):204-211.
doi pubmed - Ferri S, Muratori L, Lenzi M, Granito A, Bianchi FB, Vergani D. HCV and autoimmunity. Curr Pharm Des. 2008;14(17):1678-1685.
doi pubmed - Vergani D, Mieli-Vergani G. Autoimmune manifestations in viral hepatitis. Semin Immunopathol. 2013;35(1):73-85.
doi pubmed - Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Pileri SA, et al. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39(Suppl 1):S13-21.
doi - Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2002;97(8):2040-2045.
doi pubmed - Rajan SK, Espina BM, Liebman HA. Hepatitis C virus-related thrombocytopenia: clinical and laboratory characteristics compared with chronic immune thrombocytopenic purpura. Br J Haematol. 2005;129(6):818-824.
doi pubmed - de Almeida AJ, Campos-de-Magalhaes M, Antonietti CL, Brandao-Mello CE, da Silva MLP, de Oliveira RV, do Espirito-Santo MP, et al. Autoimmune thrombocytopenia related to chronic hepatitis C virus infection. Hematology. 2009;14(1):49-58.
doi pubmed - Chiao EY, Engels EA, Kramer JR, Pietz K, Henderson L, Giordano TP, Landgren O. Risk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infection. Arch Intern Med. 2009;169(4):357-363.
doi pubmed - Dimitroulis D, Valsami S, Stamopoulos P, Kouraklis G. Immunological HCV-associated thrombocytopenia: short review. Clin Dev Immunol. 2012;2012:378653.
doi pubmed - Zhang L, Li H, Zhao H, Ji L, Yang R. Hepatitis C virus-related adult chronic idiopathic thrombocytopenic purpura: experience from a single Chinese center. Eur J Haematol. 2003;70(3):196-197.
doi pubmed - Ramos-Casals M, Garcia-Carrasco M, Lopez-Medrano F, Trejo O, Forns X, Lopez-Guillermo A, Munoz C, et al. Severe autoimmune cytopenias in treatment-naive hepatitis C virus infection: clinical description of 35 cases. Medicine (Baltimore). 2003;82(2):87-96.
doi - Kamesaki T, Oyamada T, Omine M, Ozawa K, Kajii E. Cut-off value of red-blood-cell-bound IgG for the diagnosis of Coombs-negative autoimmune hemolytic anemia. Am J Hematol. 2009;84(2):98-101.
doi pubmed - Marks PW. Hematologic manifestations of liver disease. Semin Hematol. 2013;50(3):216-221.
doi pubmed - Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95(10):2936-2939.
doi pubmed - Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000-1007.
doi pubmed - Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15(37):4653-4658.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


