| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 4, Number 3, September 2015, pages 202-204
Management of a Patient With Subdural Hematoma Complicated by the Presence of Heparin-Induced and Suspected Concomitant Immune Thrombocytopenia: A Case Report
Roza Chairetia, d, Argyrios Gkasiamisb, Tomas L. Lindahlc
aDepartment of Molecular Medicine and Surgery, Karolinska Institutet, Solna, Stockholm, Sweden
bArgyrios Gkasiamis, Department of Internal Medicine, Hematology Section, St Goran’s Hospital, Sweden
cDepartment of Clinical and Experimental Medicine, Linkoping University, Linkoping, Sweden
dCorresponding Author: Roza Chaireti, Department of Molecular Medicine and Surgery, Karolinska Institutet, Solna, Stockholm, Sweden
Manuscript accepted for publication August 18, 2015
Short title: Thrombocytopenia in Subdural Hematoma
doi: http://dx.doi.org/10.14740/jh219w
| Abstract | ▴Top |
A patient with antiphospholipid syndrome treated with warfarin presented to the emergency department with a traumatic subdural hematoma. Two days following the evacuation of the hematoma, treatment with low molecular weight heparin (LMWH) was initiated. One week later, the patient developed new symptoms. The hematoma had relapsed and the platelet count had decreased considerably. The thrombocytopenia was diagnosed as heparin-induced and the anticoagulant treatment was discontinued. Following increase of the platelet count, treatment with fondaparinux was initiated. However, 1 day following that, the platelet count had decreased again and cross-reaction between the LMWH and fondaparinux was suspected. Due to the risk of hematoma relapse, the patient started treatment with cortisone, responding promptly. The platelet count was stabilized and the patient resumed the treatment with warfarin without complications. The thrombocytopenia was initially heparin-induced and complicated by the cross-reaction between LMWH and fondaparinux as well as the presence of suspected autoimmune thrombocytopenia, as suggested by the response to corticosteroids. This case report illustrates the difficulties in diagnosing and managing thrombocytopenia of complex etiology.
Keywords: Thrombocytopenia; Antiphospholipid syndrome; Fondaparinux
| Introduction | ▴Top |
We present the case of a patient with concomitant heparin-induced thrombocytopenia (HIT) and immune thrombocytopenia, which was further complicated by the cross-reaction between low molecular weight heparin (LMWH) and fondaparinux, leading to relapse of HIT. This case illustrates the difficulties in diagnosing and managing cases of thrombocytopenia of complex etiology as well as provides data on the controversial subject of relapsed HIT caused by fondaparinux cross-reaction.
| Case Report | ▴Top |
A 65-year-old woman presented to the emergency department (ED) of the Vrinnevi Hospital, Norrkoping, Sweden in January 2013 complaining of headache. The symptoms had started a week ago, following a falling accident and a subsequent head trauma. No other neurological symptoms were reported. The patient was under lifelong treatment with warfarin because of antiphospholipid syndrome (APS). She had suffered a non-cardioembolic ischemic stroke in 2003 and the laboratory investigation had revealed positive lupus anticoagulant twice with 12 weeks’ interval [1]. At the ED, the patient was investigated with a computed tomography (CT) of the head, which showed a chronic subdural hematoma (SDH). The prothrombin time-international normalized ratio (PT-INR) was 2.7 (recommended therapeutic interval 2 - 3) at the time of the presentation. Warfarin was reversed by administration of 1,500 U prothrombin complex concentrate and 10 mg vitamin K intravenously. The hemoglobin, creatinine, platelet count and the activated partial thromboplastin time (APTT) were normal. The patient was transferred to the Neurosurgery Clinic of the Linkoping University Hospital, Sweden where the hematoma was successfully evacuated. Thromboprophylaxis with 2,500 U of the LMWH tinzaparin was initiated on the first postoperative day, in accordance with the local routines. However, a control head CT 3 days post-operatively showed that the hematoma had increased in size and the patient was operated again. She was released from the hospital after 1 week in good condition. The platelet count was 151 × 109/L at that time (reference range: 140 - 350 × 109/L) (Fig. 1).
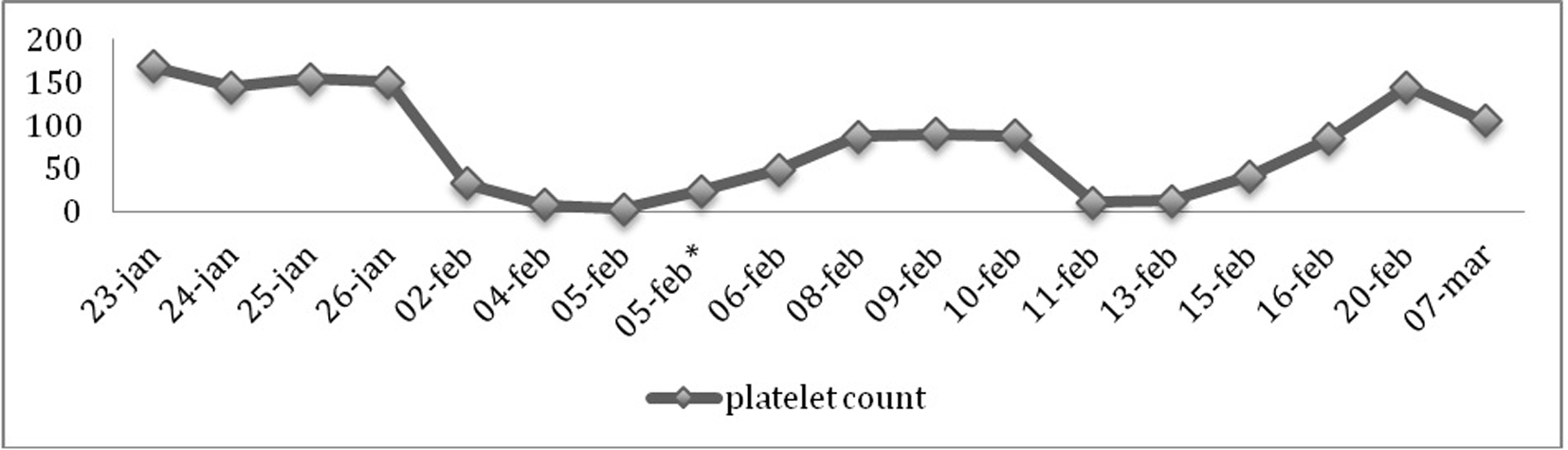 Click for large image | Figure 1. Thrombocyte count variations from the time of the patient’s first presentation at the ED until the stabilization of thrombocyte count following initiation of treatment with cortisone. 23-Jan: initial presentation at the ED (169 × 109/L). 25-Jan: initiation of treatment with LMWH (154 × 109/L). 2-Feb: second presentation at the ED (33 × 109/L). 4-Feb: discontinuation of treatment with LMWH (8 × 109/L). 5-Feb*: following thrombocyte transfusion (24 × 109/L). 10-Feb: initiation of treatment with fondaparinux (88 × 109/L). 11-Feb: discontinuation of treatment with fondaparinux (11 × 109/L). 13-Feb: initiation of treatment with cortisone (13 × 109/L). |
The patient presented again to the ED of the Vrinnevi Hospital on the day following release complaining of tingling in the right arm and leg as well as temporary expressive aphasia. A new head CT showed relapse of the SDH. The laboratory investigation revealed a platelet count of 33 × 109/L (Fig. 1). However, the thrombocytopenia was neither managed nor investigated at that moment and treatment with LMWH (at that time 4,500 U tinzaparin) continued. The patient was transferred to the Neurosurgery Clinic of the Linkoping University Hospital for a new operation. The platelet count continued to decrease with a nadir at 3 × 109/L (Fig. 1). The treatment with LMWH was discontinued. The patient was transfused with four platelet concentrates, which increased the platelet count to 24 × 109/L (Fig. 1). No bleeding symptoms were recorded. PT-INR, APTT, fibrinogen, antithrombin, creatinine and transaminases were normal. There were no signs of hemolysis or renal failure and the patient had not developed any new neurological symptoms. There were no clinical or laboratory evidence of sepsis. As it was possible that the thrombocytopenia was secondary to APS, blood tests for antiphospholipid antibodies were performed. Both the cardiolipin antibodies and beta 2 glycoprotein I antibodies were negative. The lupus anticoagulant (diluted Russell’s viper venom time) was marginally increased (1.37, reference range < 1.3).
According to the pre-test clinical score (4T score) for HIT, the patient had a moderate probability; 2 points for the timing of platelet count fall (the patient was first exposed to LMWH on January 24 and the platelet count had decreased to 33 × 109/L by February 2), 0 point for thrombosis or other sequelae and 1 point for other possible causes for thrombocytopenia (such as immune thrombocytopenia) [2]. However, it was more challenging to determine the “thrombocytopenia” parameter, as the patient’s platelet count had decreased > 50% from the initial value (151 × 109/L) by the 2 February (2 points) but then reached a nadir of < 10 (0 points) as LMWH was not discontinued. The heparin-induced thrombocyte aggregation test for circulating antibodies against heparin/platelet factor 4 (PaGIA heparin/platelet factor (PF) 4 antibody test, Bio-Rad®, Solna, Stockholm, Sweden) was positive. The IgG-specific ELISA for anti-PF4-heparin antibodies (Universitatsmedizin Greifswald, Greifswald, Germany) was positive and inhibition by high concentration of heparin was present. The heparin-induced platelet activation test (HIPA test, Universitatsmedizin Greifswald, Greifswald, Germany) was positive with platelets from four out of four blood donors. Following discontinuation of tinzaparin, the platelet count increased spontaneously to 88 × 109/L and no further transfusions were necessary (Fig. 1).
The patient’s thrombocytopenia was determined as HIT. At that point treatment with fondaparinux was initiated [3]. However, the platelet count decreased from 88 × 109/L to 11 × 109/L on the next day (Fig. 1). This was perceived as cross-reaction between LMWH and fondaparinux and the treatment was discontinued. Due to the new decrease in platelets and the risk for relapse of SDH, the patient started treatment with high-dose cortisone (100 mg orally), to which she responded promptly (Fig. 1), suggesting that there could be indeed an additional immune component in the etiology of the thrombocytopenia [4].
During the spring of 2013, the patient was treated with successively lower cortisone doses. As the platelet count had stabilized at about 150 × 109/L and the latest head CT had not shown any evidence of relapse of the SDH, it was deemed safe to re-initiate treatment with anticoagulants. According to the guidelines issued by the American Stroke Association in 2011 [5], patients with APS and non-cardioembolic stroke should be treated with warfarin. However, according to the American College of Chest Physicians guidelines from 2012 [6], patients with APS and non-cardioembolic stroke should be treated in the same way as patients without APS, i.e. with clopidogrel or aspirin/dipyridamol. In this case, warfarin was chosen over platelet inhibitors, mainly due to the possibility of reversal in case of bleeding complications. The SDH was deemed as traumatic and not warfarin-associated and warfarin was thus not contraindicated in this patient.
| Discussion | ▴Top |
HIT is a potentially life-threatening prothrombotic disorder characterized by thrombocytopenia and thrombosis. Exposure to heparin can mediate formation of pathogenic IgG antibodies that recognize complexes of PF4 and heparin on platelet surface leading to platelet activation [7]. The laboratory testing for detection of HIT antibodies includes both PF4-dependent “antigen” assays as well as platelet “activation” (functional) assays [7].
The probability of cross-reaction between fondaparinux and LMWH in the context of HIT has been a subject of debate, but it has, however, been reported before [8]. In our patient’s case, the platelets decreased again the day following exposure to fondaparinux, as is expected in the natural history of HIT when it debuts after new exposure to LMWH following previous treatment during the last 3 months [2].
This case was further complicated by the presence of suspected concomitant immune thrombocytopenia [4], although involvement of APS cannot be excluded. APS is linked to a high risk of a first and recurrent venous and/or arterial thrombotic episodes, as well as obstetric complications and is a cause of autoimmune thrombocytopenia which is treated in the same way as immune thrombocytopenia, for example corticosteroids, immunoglobulin, etc. [1]. However, the absence of new thromboembolic complications, even during the weeks when the patient was not on anticoagulant treatment, as well as the low titers of antiphospholipid antibodies, speaks against an active APS. In patients with APS, the PF4/heparin antibodies can be a false positive finding due to the possibility of circulating autoantibodies against PF4 [9]; however, in this case both the functional assay (HIPA) and the ELISA were also positive.
The platelets increased following concomitant discontinuation of fondaparinux and initiation of cortisone therapy; individual effect evaluation is thus difficult. However, the promptness of increase following cortisone treatment, considering the long half-life of fondaparinux [10] strongly indicates that immune thrombocytopenia was part of the cause. Two cases of HIT followed by immune thrombocytopenia have been previously reported [11]; however, the precise pathophysiological mechanisms are unclear.
In conclusion, the thrombocytopenia in this patient was initially caused by HIT and further complicated by cross-reaction with fondaparinux and the presence of suspected immune thrombocytopenia. Thrombocytopenia is not uncommon in seriously ill patients and its cause can be multifactorial [12]. It is imperative that the clinician keeps that in mind since, following correct and prompt diagnosis, the patient can be treated successfully.
Conflict of Interest
The authors declare no conflict of interest.
Abbreviations
HIT: heparin-induced thrombocytopenia; LMWH: low molecular weight heparin; APS: antiphospholipid syndrome; ED: emergency department; CT: computed tomography; SDH: subdural hematoma; PT-INR: prothrombin time-international normalized ratio; APTT: activated partial thromboplastin time; PF4: platelet factor 4
| References | ▴Top |
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306.
doi pubmed - Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759-765.
doi pubmed - Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S-530S.
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495-520.
doi pubmed - Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1):227-276.
doi pubmed - Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e601S-636S.
- Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2003:497-519.
doi - Pistulli R, Oberle V, Figulla HR, Yilmaz A, Pfeifer R. Fondaparinux cross-reacts with heparin antibodies in vitro in a patient with fondaparinux-related thrombocytopenia. Blood Coagul Fibrinolysis. 2011;22(1):76-78.
doi pubmed - Pauzner R, Greinacher A, Selleng K, Althaus K, Shenkman B, Seligsohn U. False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Thromb Haemost. 2009;7(7):1070-1074.
doi pubmed - http://www.rxlist.com/arixtra-drug/clinical-pharmacology.htm
- Waheed F, Naseer N, Ahmed T, Nelson JC. Two patients with heparin-induced thrombocytopenia followed by idiopathic (immune) thrombocytopenic purpura: case report. Am J Hematol. 2003;73(4):290-293.
doi pubmed - Parker RI. Etiology and significance of thrombocytopenia in critically ill patients. Crit Care Clin. 2012;28(3):399-411, vi.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


