| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 1, Number 1, February 2012, pages 32-35
Massive SVC Thrombus in a Woman With Heterozygous Methyl-Tetrahydrofolate Reductase A1298C Genetic Mutation
Abdelkarim Wanessa, d, Hiba Chehadeb, Mohamed El Sheikhc
aConsultant, Medicine Institute, Sheikh Khalifa Medical City, Abu Dhabi, UAE
bResident, Medicine Institute, Sheikh Khalifa Medical City, Abu Dhabi, UAE
cSpecialist, Medicine Institute, Sheikh Khalifa Medical City, Abu Dhabi, UAE
dCorresponding author: Abdelkarim Waness, Consultant, Internal Medicine, Sheikh Khalifa Medical City, P.O. Box: 51900, Abu Dhabi, UAE
Manuscript accepted for publication February 14, 2012
Short title: Massive SVC Thrombus
doi: https://doi.org/10.4021/jh15w
| Abstract | ▴Top |
Some genetic mutations are known to be thrombophilic. Methyl-tetrahydrofolate reductase (MTHFR) mutations are among the most recently described. Further, large Superior Vena Cava thrombi are a rare occurrence. They are usually discovered in the setting of invasive venous procedures or in patients afflicted with malignancies. Their presentation can be dramatic in the form of Superior Vena Cava Syndrome requiring urgent therapeutic intervention. We present a unique case of a covert large Superior Vena Cava thrombus in a woman diagnosed with heterozygous Methyl-tetrahydrofolate reductase A1298C genetic mutation. She presented with symptoms of bilateral pulmonary embolism requiring urgent thrombolytic intervention. A discussion about the prevalence of Superior Vena Cava thrombi, their risk factors, their possible presentations, diagnostic modalities and therapeutic options follows the presentation.
Keywords: Methyl-tetrahydrofolate reductase A1298C mutation; Superior vena cava thrombus; Pulmonary embolism; Computerized tomography scan; Thrombolysis; Anticoagulation
| Introduction | ▴Top |
SVC thromboembolism is a rare form of venous thromboembolic disease. Classic forms of this condition are described in patients suffering from malignancies, thrombophilia, or following central venous catheter insertion. Methyl-tetrahydrofolate reductase is an enzyme responsible for re-methylayion of homocysteine to methionine. This enzyme can have two mutations-C677T and A1298C- which can lead to thrombophia and fetal loss usually in the homozygous mutations. The occurrence of recurrent VTE in patients with heterozygous Methyl-tetrahydrofolate reductase A1298C genetic mutation is extremely rare.
| Case Report | ▴Top |
A 47 year- old Caucasian female was brought to the department of Emergency Medicine with one-day history of chest pain, worsening dyspnea and presyncope. She was treated 12 years ago for lower extremity deep venous thrombosis while pregnant. She denied any prior spontaneous abortions. The patient is also known to have premature ovarian failure and has never received estroprogestative treatment. She was not taking any medication when seen. Physical examination revealed a diaphoretic patient in clear respiratory distress. Her vital signs were: heart rate 128 beats/minute, respiratory rate 32 breaths/minute, blood pressure was 126/72 mmHg, and temperature 36.8 °C. She was normocephalic with no evidence of facial edema, or cyanosis. Her neck veins were not distended. Her upper extremities were normal in size with no obvious discoloration. Chest examination revealed tachycardia and reduced breath sounds bilaterally. She has extensive varicose veins below knees bilaterally without superficial skin or deep calf tenderness. The circumference of both legs was equal. Arterial blood gases on room air showed: pH = 7.38, PO2 = 58 mm Hg and oxygen saturation at 90%. D-Dimer level was elevated at 7.4 mcg/mL. Serum troponin-I, electrolytes, blood counts, coagulation profile, kidney and liver functions were normal. Electrocardiogram shows only sinus tachycardia. Chest X-ray was unremarkable. A quick on-site trans-thoracic echocardiogram revealed mild elevation of pulmonary artery pressure at 42 mmHg with normal left ventricular function. Chest Computed Tomography - Pulmonary Angiogram showed extensive pulmonary embolism seen in both pulmonary arteries extending into upper and lower lobe branches with mild enlargement of the right ventricle. This imaging modality also accidentally revealed severe incomplete filling of the superior vena cava extending upward into the right innominate vein (Fig. 1). The patient was emergently treated with intravenous alteplase and transferred to the Intensive Care Unit. A Doppler scan of her legs showed normal blood flow with normal velocities and no evidence of deep thrombosis. The patient was also started on therapeutic dose of low-molecular weight heparin. After clinical improvement, she was transferred to the ward where she had successful anticoagulation bridging to warfarin. Thrombophilia panel was negative. She was however found to be heterozygous for the Methyl-Tetrahydrofolate Reductase (MTHFR) A1298C genetic mutation. The patient was discharged home and has been regularly following with the anticoagulation clinic.
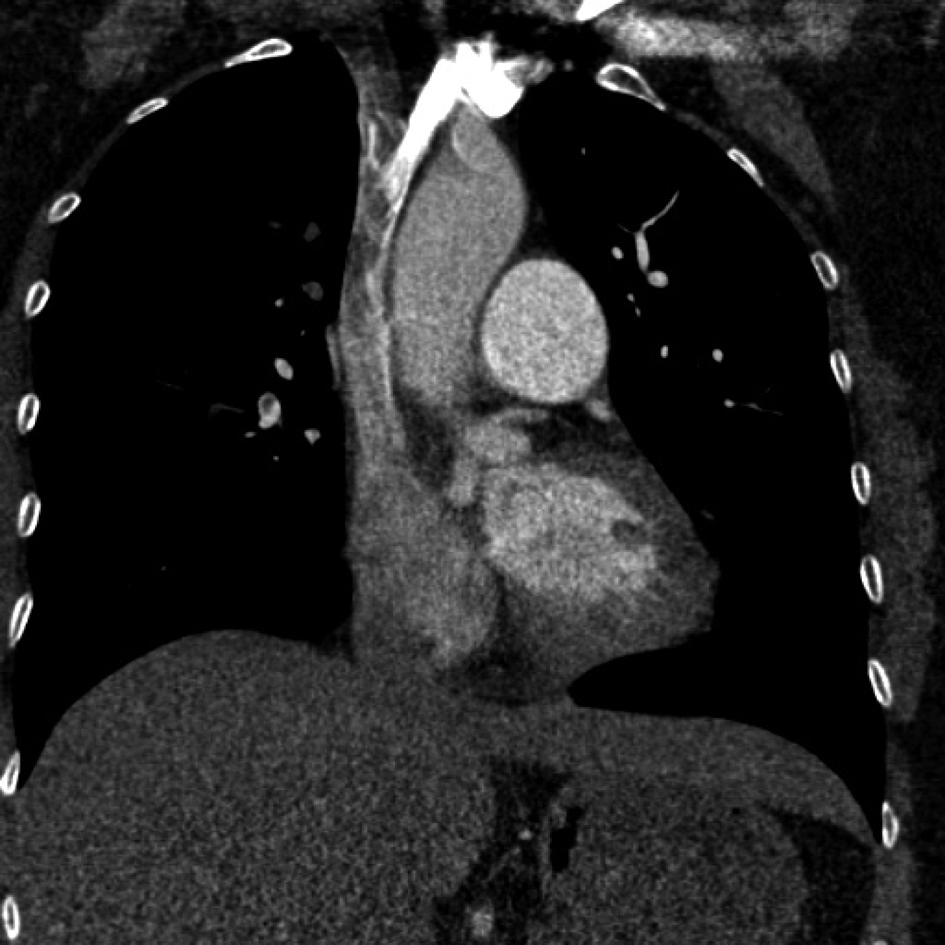 Click for large image | Figure 1. Large filling defect of the Superior Vena Cava extending upward to the Right Innominate Vein after contrast infusion. |
| Discussion | ▴Top |
Venous thromboembolism (VTE) remains prevalent and can be deadly despite recent diagnostic and therapeutic advances. According to the American College of Physicians, the yearly incidence of VTE in the United States alone is more than a half million cases, with up to 200,000 of them ending up dying from it [1]. Thromboembolic disease located at the Superior Vena Cava (SVC) and brachiocephalic veins (BCV) is a very rare form of VTE. According to Oymak and colleagues, its prevalence is 0.03% among hospitalized patients [2]. This recent Turkish study confirmed that SVC and BCV thromboses are usually secondary to specific risk factors namely: malignancy, thrombophilia, chronic disorders, or iatrogenic following insertion of peripheral venous lines or central venous catheters (CVC). It has been shown that the location of CVC is crucial to the development of SVC thrombus. Indeed, Cadman et al showed that proximally-placed CVC (proximal third SVC or thoracic inlet veins) are 16 times more likely to thrombose than the ones placed distally(distal third of the SVC or right atrium) [3]. In case of malignancy, the pathogenesis of thrombus formation in the SVC can be complex related to many factors such as humoral ones, mechanic obstruction, or even fibrosis caused by radiotherapy [4]. Collagen vascular diseases and Behçet’s disease are among rare risk factors for SVC thrombosis [5]. It is currently well-documented that certain inherited genetic mutations, such as factor V Leiden, factor V Hong Kong, or factor II G20210A can predispose to venous or arterial thrombosis [6]. Methyl-tetrahydrofolate reductase is an enzyme responsible for re-methylation of homocysteine to methionine [7]. Two major mutations - C677T and A1298C - were discovered in many ethnic groups [8]. These two MTHFR mutations are prevalent in the Middle East. In a large series of 188 patients, Tug et al reported MTHFR C677T and A1298C frequencies of 30.4% and 39.1% respectively. The former was frequently associated with pulmonary embolism (37%), while the latter was pervasive in patients having recurrent spontaneous abortions (55.9%) [9]. These thrombophilic complications are described mostly in homozygous subjects. Our patient however was found to be heterozygous for the MTHFR A1298C genetic mutation. The uniqueness of her presentation is not only the SVC location but also her thrombophilic inclination (two documented episodes) with only heterozygous status.
Symptomatology of SVC thrombosis stems from the progressive increase in the venous pressure within this large vessel. The hallmark of this presentation is “SVC syndrome” with its varying degree of severity. Indeed, neck and facial edema with venous hyperemia can be observed. These changes can manifest themselves in the upper extremities also. Increasing pressure can compromise breathing and swallowing. Patients can present with dyspnea, hoarsness, or dysphagia. If the venous pressure is persistently and extremely elevated, it can cause cerebral edema resulting in confusion, coma and even death [10]. Our patient sought medical care relatively early and did not have any verifiable symptom of SVC syndrome. She had obvious dyspnea at presentation explained by her massive pulmonary embolism. This complication is encountered with more than a third (36%) of patients with SVC thrombus [2].
SVC thrombosis can be relatively easy to diagnose if presented with the clinical picture of SVC syndrome. In the absence of such presentation or symptoms of pulmonary embolism, its diagnosis can be more elusive. Recent guidelines, such as the one established by the American Academy of Family Physicians and the American College of Physicians, stress the importance of adhering to clinical prediction based good-quality evidence in the diagnosis of VTE [11]. The Wells prediction rules were validated to measure the probability of VTE and therefore guide further investigations. D-dimer assays, a highly sensitive but nonspecific plasma coagulation marker, are helpful in confirming acute deep venous thrombosis (DVT) [12]. Nowadays, there is a formidable battery of imaging techniques available for diagnosing VTE. In case of SVC thrombosis, computer axial tomography (CT) is readily available and easy to perform. Magnetic Resonance Angiography imaging (MRA) offers another solid way for the evaluation of the SVC [13]. Occasionally, transesophageal echocardiography (TEE) can diagnose SVC thrombosis [14]; the radiologic gold-standard test for this condition remains contrast venography however [11].
Many therapeutic alternatives are currently available for the treatment of VTE. They are usually well-detailed in evidence-based guidelines recommended by renown professional medical organizations, such as the “antithrombotic and thrombolytic therapy 8th edition: ACCP guidelines” by the American College of Chest Physicians [15]. These recommendations can generally apply to SVC thrombus. The treatment mainstay is made of low molecular weight heparin or vitamin K antagonists such as warfarin. In life-threatening situations however, thrombolytic therapy is used to dissolve massive clots obstructing the superior vena cava and its tributaries [4]. Placing SVC filters is very controversial and carries the risk of severe complications such as cardiac tamponade, pneumothorax, SVC perforation, or filter migration [16]. Occasionally, and depending on the underlying condition, other rare therapeutic modalities might be undertaken. Examples include: SVC metallic stent placement [17], or surgical SVC reconstruction using autologous tissue [18].
It is always important to apply deep venous thrombosis preventive measures, according to current guidelines, especially for patients at higher risk for VTE [19]. Finally, some authors advocate thrombophilia screening in selected populations, such as Eastern Mediterranean or Hispanic, where these forms of genetic mutations are prevalent [8-20].
Conclusion
Superior Vena Cava thrombosis is an extremely rare medical occurrence. It is usually present in the setting of malignancy, invasive catheterization, or thrombophilia. Methyl-tetrahydrofolate reductase A1298C genetic mutation, especially in its homozygous form, can rarely and potentially cause SVC thrombosis. Its manifestations vary from completely silent to SVC syndrome. It has high risk of pulmonary embolism requiring thrombolysis in life-threatening situations. Long-term anticoagulation remains the therapeutic cornerstone for this relatively severe pathology. Preventing thromboembolism is a high medical priority, while screening for thrombophilia might be beneficial in selected populations.
Conflict of Interest
None.
Funding Source
Nil.
| References | ▴Top |
- American College of Physicians. http://www.medscape.org/viewarticle/551627 [cited May 2011].
- Oymak FS, Buyukoglan H, Tokgoz B, Ozkan M, Tasdemir K, Mavili E, Gulmez I, et al. Prevalence of thromboembolic disease including superior vena cava and brachiocephalic veins. Clin Appl Thromb Hemost. 2005;11(2):183-189.
pubmed doi - Cadman A, Lawrance JA, Fitzsimmons L, Spencer-Shaw A, Swindell R. To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 2004;59(4):349-355.
pubmed doi - Salmi R, Gaudenzi P, Di Todaro F, Morandi P, Nielsen I, Manfredini R. Massive thrombosis of brachiocephalic veins and superior vena cava syndrome in a patient with non-small cell lung cancer treated with the epidermal growth factor receptor inhibitor erlotinib. Clin Drug Investig. 2007;27(7):499-503.
pubmed doi - Roguin A, Edelstein S, Edoute Y. Superior vena cava syndrome as a primary manifestation of Behcet's disease. A case report. Angiology. 1997;48(4):365-368.
pubmed doi - Dolek B, Eraslan S, Eroglu S, Kesim BE, Ulutin T, Yalciner A, Laleli YR, et al. Molecular analysis of factor V Leiden, factor V Hong Kong, factor II G20210A, methylenetetrahydrofolate reductase C677T, and A1298C mutations related to Turkish thrombosis patients. Clin Appl Thromb Hemost. 2007;13(4):435-438.
pubmed doi - Altomare I, Adler A, Aledort LM. The 5, 10 methylenetetrahydrofolate reductase C677T mutation and risk of fetal loss: a case series and review of the literature. Thromb J. 2007;5:17.
pubmed - Chowdary D, Streck D, Schwalb MN, Dermody JJ. High incidence of two methylenetetrahydrofolate reductase mutations (C677T and A1298C) in Hispanics. Genet Test. 2003;7(3):255-257.
pubmed doi - Tug E, Aydin H, Kaplan E, Dogruer D. Frequency of genetic mutations associated with thromboembolism in the Western Black Sea Region. Intern Med. 2011;50(1):17-21.
pubmed doi - Taguchi J, Kinoshita I, Akita H. [Superior vena cava syndrome]. Gan To Kagaku Ryoho. 2011;38(4):518-523.
pubmed - Qaseem A, Snow V, Barry P, Hornbake ER, Rodnick JE, Tobolic T, Ireland B, et al. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5(1):57-62.
pubmed doi - Meissner MH, Zierler BK, Bergelin RO, Chandler WC, Manzo RA, Strandness DE, Jr. Markers of plasma coagulation and fibrinolysis after acute deep venous thrombosis. J Vasc Surg. 2000;32(5):870-880.
pubmed doi - Tomasian A, Lohan DG, Laub G, Singhal A, Finn JP, Krishnam MS. Noncontrast 3D steady state free precession magnetic resonance angiography of the thoracic central veins using nonselective radiofrequency excitation over a large field of view: initial experience. Invest Radiol. 2008;43(5):306-313.
pubmed doi - Barbeito A, Bar-Yosef S, Lowe JE, Atkins BZ, Mark JB. Unusual cause of superior vena cava syndrome diagnosed with transesophageal echocardiography. Can J Anaesth. 2008;55(11):774-778.
pubmed doi - Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ. Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):71S-109S.
pubmed doi - Owens CA, Bui JT, Knuttinen MG, Gaba RC, Carrillo TC. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(6):779-787.
pubmed doi - de Gregorio Ariza MA, Gamboa P, Gimeno MJ, Alfonso E, Mainar A, Medrano J, Lopez-Marin P, et al. Percutaneous treatment of superior vena cava syndrome using metallic stents. Eur Radiol. 2003;13(4):853-862.
pubmed - Wada N, Masudo K, Hirakawa S, Woo T, Arai H, Suganuma N, Iwaki H, et al. Superior vena cava (SVC) reconstruction using autologous tissue in two cases of differentiated thyroid carcinoma presenting with SVC syndrome. World J Surg Oncol. 2009;7:75.
pubmed - Kanaan AO, Silva MA, Donovan JL, Roy T, Al-Homsi AS. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29(11):2395-2405.
pubmed doi - Eid SS, Shubeilat T. Prevalence of factor V Leiden, prothrombin G20210A, and MTHFR G677A among 594 thrombotic Jordanian patients. Blood Coagul Fibrinolysis. 2005;16(6):417-421.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


