| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 5, Number 1, March 2016, pages 1-7
Evaluation of an Outpatient Model for Treatment of Acute Myeloid Leukemia
Andrew Awa, Mitchell Sabloffa, b, c, e, Dawn Shepparda, b, c, d, David Allana, c, d, Harold Atkinsa, c, d, Isabelle Bence-Brucklera, c, d, Carolyn Faughta, Lothar Huebscha, c, Jason Taya, c, d, Kate Dukec, Timothy Ramsayd, Chris Bredesona, c, d
aDivision of Hematology, The Ottawa Hospital, Ottawa, ON, Canada
bThe Ottawa Hospital Leukemia Program, Ottawa, ON, Canada
cThe Ottawa Hospital Blood and Marrow Transplant Program, Ottawa, ON, Canada
dThe Ottawa Hospital Research Institute, Ottawa, ON, Canada
eCorresponding Author: Mitchell Sabloff, Division of Hematology, Department of Medicine, The Ottawa Hospital, 501 Smyth Road, Ottawa, ON, K1H 8L6, Canada
Manuscript accepted for publication December 15, 2015
Short title: Outpatient Management of Acute Leukemia
doi: http://dx.doi.org/10.14740/jh235w
| Abstract | ▴Top |
Background: In January 2012, our center developed an ambulatory model for acute myeloid leukemia (AML) patients receiving post-induction chemotherapy. The purpose of this study was to evaluate the feasibility and safety of the outpatient leukemia program at our center.
Methods: A retrospective review of all consecutive AML patients receiving a first cycle of consolidation chemotherapy in the outpatient program in 2012 was compared to similarly managed patients primarily in the inpatient setting in 2010.
Results: The 2012 cohort spent more days as outpatients in comparison to the 2010 cohort (median (range): 15.5 (0 - 27) vs. 0 (0 - 10), days, P = 0.002). There was no difference between the two cohorts in terms of median overall observation time (time from start of chemotherapy until discharged to clinic), transfusion requirements, days spent neutropenic or days spent febrile. There were no documented episodes of Clostridium difficile, clinically significant bleeding, venous thromboembolism, or death in either cohort.
Conclusions: Outpatient management of AML patients receiving post-induction chemotherapy was feasible in this group of carefully selected individuals, liberating limited and costly inpatient resources for more appropriate patients.
Keywords: Acute myeloid leukemia; Consolidation chemotherapy; Antineoplastic combined chemotherapy protocols; Outpatients; Ambulatory care; Multidisciplinary care
| Introduction | ▴Top |
Standard treatment for AML includes induction chemotherapy, typically with an anthracycline and cytarabine based regimen. With such regimens, 75-80% of patients achieve a complete morphologic remission (CR) [1]. Although high initial CR rates are achieved, disease relapse is virtually unavoidable without subsequent post-induction therapy. Standard post-induction treatment consists of either consolidation chemotherapy or an allogeneic hematopoietic stem cell transplantation (HSCT) [2]. Because of the treatment intensity and potential complications of resultant prolonged pancytopenia, post-induction chemotherapy has traditionally been managed in an inpatient setting. In the conventional model, patients are hospitalized for the duration of their chemotherapy regimen and until their blood cell counts have recovered.
Management of malignancy in the outpatient setting has become increasingly common. While limitations on health care expenditures have played a role in this transition, improved abilities to provide supportive care, to monitor patients regularly, and to expedite admission to hospital when necessary have also facilitated change in practice. Outpatient management of solid tumor malignancies is well described [3]. Ambulatory programs for high-dose chemotherapy followed by autologous hematopoietic stem cell rescue represent a viable option [4-6]. In selected patients with AML, outpatient administration of consolidation chemotherapy cycles has also been reported [7-10].
In January 2012, The Ottawa Hospital Leukemia Program (TOHLP) developed an outpatient management model for AML patients receiving post-induction chemotherapy, a transition facilitated by the creation of dedicated outpatient resources and the success of the pre-existing outpatient blood and marrow transplant program. The experience of AML patients receiving outpatient consolidation therapy at our institution has not yet been formally described. The purpose of this study was to evaluate the feasibility and safety of the outpatient leukemia program at The Ottawa Hospital (TOH), as compared to our previous inpatient model.
| Materials and Methods | ▴Top |
A retrospective review of all consecutive AML patients receiving a first cycle of consolidation chemotherapy in the outpatient program from January to December 2012 was compared to similarly managed patients primarily in the inpatient setting from January to December 2010. Patients were identified from a database maintained by TOHLP. Eligible patients were those who achieved a first complete remission (CR1) after induction chemotherapy for AML who went on to receive at least one cycle of consolidation chemotherapy at TOH. A local policy was created in January 2009 for the use of prophylactic antimicrobials in high-risk neutropenic patients with hematologic malignancies. The study dates were chosen to ensure patients in both groups were managed in a similar fashion with regard to antimicrobial use, after this policy change was implemented [11]. The study was approved by the Ottawa Health Science Network Research Ethics Board.
All patients had a confirmed diagnosis of AML prior to induction chemotherapy through bone marrow aspirate and biopsy, with routine Giemsa banding cytogenetic analysis and flow cytometry requested on all samples. Patients with normal karyotype had standard molecular analysis requested to look for FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) and nucleophosmin 1 (NPM1) mutations. Cytogenetic and molecular analyses were used to risk stratify patients and guide selection of chemotherapy regimens, in accordance with published guidelines [2]. Patients with favorable risk disease generally received consolidation with high-dose cytarabine (HiDAC). Those with intermediate and adverse risk disease were administered combination chemotherapy with an anthracycline agent and cytarabine. All patients received chemotherapy through central venous access.
Treatment protocols and procedures
TOH is a regional tertiary care referral center for a catchment population of approximately 1 million and is the sole provider of leukemia care for this population.
Patients in the outpatient program have daily assessments, including on weekends, until blood cell count recovery and until judged safe enough to be discharged for subsequent follow-up in clinic. Daily visits include physical assessment, measurement of vital signs, laboratory investigations, and administration of prescribed therapy including, but not limited to, chemotherapy, intravenous (IV) antibiotics and IV hydration. To qualify for the outpatient program, a patient must be deemed stable by the leukemia team, have an available caregiver 24 h/day, live within 60 min from the hospital, and demonstrate the ability to participate in self-care activities such as temperature taking. Patient preference is incorporated into the decision making process. Patients with medical complications can be transferred to the inpatient setting for more intensive nursing and medical care. This transfer occurs as a smooth process directly to the hematology ward, usually bypassing the need for assessment in the emergency department (ED). Patients are provided dedicated contact information to access the on-call hematologist via telephone for issues that occur at night. Patients transferred to the inpatient setting may be transferred back to the outpatient program if their care requirements decrease, until they are ready for discharge to clinic. The cost of transportation, parking, meals and accommodation, as well as the dispensing of prescription non-intravenous medication is not provided by TOH.
All patients, regardless of inpatient or ambulatory setting, receive the same prophylactic antimicrobial coverage and supportive care. Patients are administered prophylactic ceftriaxone 1 g intravenously on a daily basis when the absolute neutrophil count is less than or equal to 0.5 × 109/L or the total white blood cell count is less than or equal to 1 × 109/L, in the absence of fever. Those with fever are administered broader spectrum antibiotics and are managed as inpatients. Patients are administered prophylactic acyclovir at a total daily dose of 800 mg orally or 400 mg intravenously, fluconazole 400 mg orally once daily, and trimethoprim/sulfamethoxazole two single-strength tablets orally twice weekly. Irradiated packed red blood cells (PRBCs) and platelet concentrates are used for all transfusions. Two units of PRBCs are administered if the morning hemoglobin is less than 80 g/L. Platelet support is given prophylactically if the morning platelet count is below 20 × 109/L.
Outcomes and definitions
Total number of days observed for first cycle of consolidation chemotherapy is defined as follows: for those patients in the outpatient program, the date of first visit to the outpatient bed until date discharged to clinic (including any days spent as an admitted inpatient in between those two dates); and for those treated as admitted inpatients, the date of admission to the hematology ward until the date discharged to clinic. Data were collected during dates observed for first cycle of consolidation chemotherapy or a total follow-up period of 30 days, whichever was longer.
Days spent neutropenic are defined as those days during which a patient had an absolute neutrophil count of less than 0.5 × 109/L. Days spent febrile were those days during which a patient had documented fever, defined as a temperature greater than 38.3 °C on one occasion or persistent temperature of 38.1 °C. Count recovery was defined as the first day with an absolute neutrophil count greater than or equal to 0.5 × 109/L and platelet count greater than or equal to 20 × 109/L for 3 days, without transfusion.
Data collection and analysis
Relevant demographic, clinical and laboratory characteristics were collected, including data on length of stay and proportion of days spent as an outpatient, comorbidities as defined by the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) [12], chemotherapy regimen administered, blood count recovery, PRBC transfusion requirements, platelet transfusion requirements, and adverse events of infection, documented bacteremia confirmed with microbiological testing, venous thromboembolism confirmed with diagnostic imaging, clinically significant bleeding [13] or death. Statistical analysis was facilitated by the standard software package SAS (Version 9.2, Statistical Analysis System Inc.). Measures of central tendency and dispersion were applied to the study patients’ demographic data. The two-tailed Wilcoxon rank-sum test was used to compare median values between the two cohorts.
| Results | ▴Top |
Sixty patients with AML were identified over the study period: 33 consecutive patients were treated for AML from January to December 2012, and 27 consecutive patients were treated for AML from January to December 2010 (Fig. 1). Of the 33 patients with AML identified in 2012 (Fig. 1a), 18 patients were evaluable (hereafter referred to as the 2012 cohort), and of the 27 patients with AML identified in 2010 (Fig. 1b), 11 patients were evaluable (hereafter referred to as the 2010 cohort).
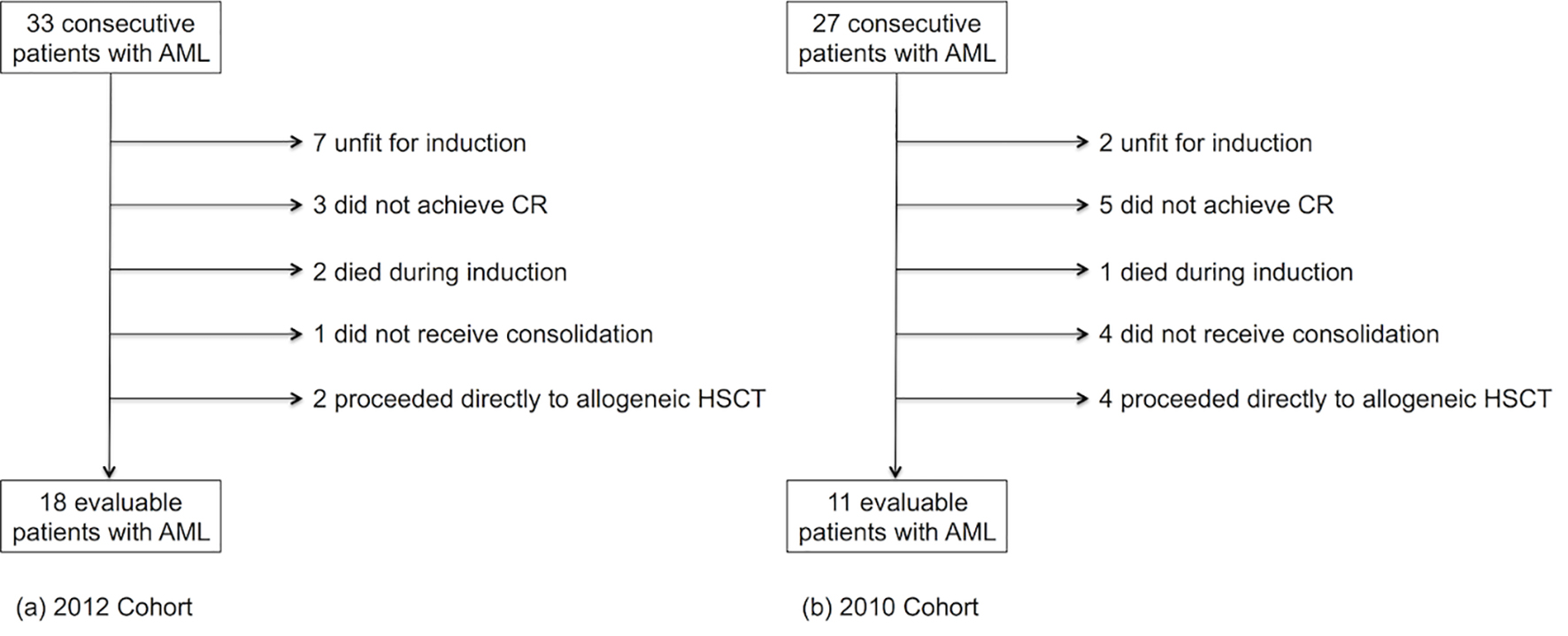 Click for large image | Figure 1. Number of evaluable patients with acute myeloid leukemia included in the (a) 2012 cohort and the (b) 2010 cohort. CR: complete morphological remission; HSCT: hematopoietic stem cell transplantation. |
Baseline characteristics of patients included in the analysis are summarized in Table 1. Baseline blood counts between the 2012 and 2010 cohorts were similar except for median platelet count at diagnosis (median (range): 42 (10 - 183) vs. 101 (15 - 307), 109/L, P = 0.028). One patient in each cohort required a second cycle of chemotherapy to achieve CR1. The majority of patients were administered another course of combination chemotherapy with an anthracycline and cytarabine as their first cycle of consolidation therapy, whereas the two patients that required re-induction were administered the chemotherapy regimen that achieved CR1. The HCT-CI could not be consistently calculated in all patients included in the study due to limitations in recording of comorbidity data. As such, we chose not to calculate the HCT-CI score retrospectively due to concerns about score accuracy.
 Click to view | Table 1. Baseline Patient Characteristics, Induction and Consolidation Regimens |
Patients spent more days as outpatients in 2012 as compared to 2010 (median (range): 15.5 (0 - 27) vs. 0 (0 - 10), days, P = 0.002) (Table 2). As expected, patients in the 2012 cohort spent more days as outpatients while neutropenic than those in the 2010 cohort (median (range): 7 (0 - 21) vs. 0 (0 - 10) days, P = 0.024). There was no difference in the total number of days observed for first cycle of consolidation chemotherapy (i.e. time from start of chemotherapy until discharged to clinic) between the two cohorts. There was no difference between the two cohorts in terms of median number of days spent neutropenic (16 vs. 14), days spent febrile (1 vs. 1), PRBC units transfused (2 vs. 2), adult pools of platelets transfused (0 vs. 0), or blood counts at discharge.
 Click to view | Table 2. Days Observed for First Cycle of Consolidation Chemotherapy, Days Spent Neutropenic, Days Spent Febrile, and Blood Counts Discharge |
In the 2012 cohort, four patients spent the entire observation period as outpatients, and four patients were admitted for the entire observation period. The most common reasons for transfer from the outpatient program to the ward were fever (6/18), poor oral intake (1/18), and diarrhea (1/18). While the majority of the patients in the 2010 cohort spent their entire observation period as inpatients, two of the 11 patients spent a number of days as outpatients. These two patients had daily assessments in the medical day care unit (MDCU), an outpatient day-unit used for patients with a variety of hematologic disorders. Both of these patients were transferred to the inpatient hematology ward during their observation period because of fever, and remained inpatients until discharged for follow-up in clinic.
One patient in the 2012 cohort required a 1-day admission to the intensive care unit (ICU) for hypotension. The patient was an inpatient on the hematology ward at the time of transfer to the ICU, and was transferred back to the ward in stable condition without requiring vasopressors or inotropic support. A coagulase-negative Staphylococcus (CoNS) infection was identified 5 days after the patient returned to the hematology ward. Five of the 18 (28%) evaluable patients in the 2012 cohort, and one of the 11 (9%) evaluable patients in the 2010 cohort had a documented episode of bacteremia. In the 2012 cohort, these episodes was comprised of two clinically insignificant cultures (Bacillus and Corynebacterium respectively), one gram-negative culture that resolved with broadened antimicrobials, one Pseudomonas aeruginosa culture associated with a rectal abscess that did not require procedural intervention and was cleared with antimicrobials alone, and the one CoNS culture noted above. In the 2010 cohort, the one episode of bacteremia corresponded to a clinically insignificant CoNS culture. There were no documented episodes of Clostridium difficile infection, clinically significant bleeding, venous thromboembolism, or death in either cohort.
| Discussion | ▴Top |
Data from this single institution study demonstrate that outpatient management of AML patients receiving consolidation chemotherapy is feasible in carefully selected individuals. Patients managed in our institution’s innovative model spent significantly more days as ambulatory patients compared to those supervised in the traditional manner. This model allows inpatient beds to be used to care for other patients requiring admission to hospital, and also permits quicker transfer of patients admitted from the ED to the hematology ward. A total of 243 inpatient bed days were saved amongst the 18 patients in the 2012 cohort. Our audit demonstrates that the outpatient management model is safe, with no major unmanageable complications over that of an inpatient model. The results of our review are consistent with those from other centers [7-10].
In our study, two out of the 11 patients in the 2010 cohort were partially managed on an outpatient basis, through the use of the MDCU. However, ongoing routine use of the MDCU was not feasible for post-induction chemotherapy in AML patients because of limited space, which resulted in delays in initiating chemotherapy on schedule. The creation of dedicated resources to facilitate the outpatient program ensures that the MDCU is available for patients with other hematologic malignancies requiring transfusional support, hydration, and chemotherapy administration. While one patient in the 2012 cohort required a brief ICU admission, this patient had already been transferred from the outpatient program to the hematology ward and was an admitted inpatient at the time of the ICU transfer. This patient had been recognized through daily outpatient assessment as having heavier care needs and was appropriately transferred to the inpatient ward in a timely fashion before requiring the ICU transfer. The ICU transfer occurred 48 h after transfer from the outpatient program to the hematology ward, highlighting the safety of the program in that even critically ill patients could be managed in an appropriate manner.
In the 2012 cohort, the median number of days observed in the program for first cycle of consolidation chemotherapy was 26.5 days, with a range of 20 - 108 days (Table 2). This wide range was driven by a single outlier, a patient who began consolidation therapy on an outpatient basis and who was transferred to the hematology ward after 7 days for poor oral intake and subsequently spent 101 days as an inpatient. This patient developed refractory thrombocytopenia delaying discharge to clinic, eventually required a thrombopoietin receptor agonist, and remains in remission at the time of this writing. Complications arising from consolidation chemotherapy do occur and it is not unexpected to have observed a patient requiring a longer than average hospital stay.
An estimated cost savings based on a median of 15 inpatient admission days saved per AML patient receiving consolidation chemotherapy could be predicted at $18,600 CAD (approximately $1,240 CAD saved per patient per day; based on the basic rate for standard ward accommodation of $1,860 CAD per day and an estimated cost per patient per day in the outpatient setting of one-third of the standard ward bed rate). The Canadian Cancer Society reported an annual incidence of AML of 1,215 Canadians in 2010 [14]. If ambulatory management models for consolidation chemotherapy were universally employed across the country, such a strategy would significantly decrease in-hospital expenditures to the Canadian health care system for a large proportion of patients with AML.
There are limitations to our study. First, the sample size of the study is small. However, based on our catchment population, the incidence of AML in both cohorts is consistent with published data [15-17]. Second, this is a retrospective cohort study and the results may be subject to bias or incomplete information. We attempted to minimize selection bias by including all consecutive patients with AML who received consolidation chemotherapy at our institution during the pre-defined study dates. We selected adverse events that were easily adjudicated. For example, presence or absence of bacteremia was a binary event that was consistently accessible on all patients, and all compression ultrasound and computed tomography scan reports performed during the defined follow-up period were reviewed for each patient to rule out objectively diagnosed venous thromboembolic events. Third, there are limitations in determining comorbid conditions retrospectively. Although designed for use in the HSCT population, the HCT-CI is also useful in prognosticating AML patients [18, 19]. Unfortunately, comorbidity data were not systematically reported in the charts of all patients included in our study. Significant comorbidities were included in admission notes, but there does not currently exist a standardized record of comorbidities on each leukemic patient. This audit identifies a practice gap to improve routine documentation of comorbidity data in all patient charts, which could be facilitated by using a checklist of HCT-CI items. Additionally, data on patient preferences and perceptions were not gathered due to the retrospective design, factors that can play important roles in the safe and effective transition to an ambulatory model [20]. Finally, a formal economic analysis of the absolute cost savings was not performed due to unavailability of the primary financial data and the analysis was performed from the health-care provider’s perspective. Such an analysis does not incorporate cost to patients and caregivers. A broader, societal, perspective along with measurement of prospective quality of life data would be an important consideration in future studies.
Conclusions
In this group of patients with AML receiving post-induction chemotherapy, this study suggests that outpatient management is safe, freeing up inpatient resources. This management strategy is facilitated by dedicated outpatient space and the ability to safely and efficiently transfer patients to the hematology ward when required.
Acknowledgement
We acknowledge Leah Shaw’s assistance in maintaining TOHLP database.
Grant Support
None.
Financial Disclosures
There are no sources of outside support to disclose.
Abbreviations
AML: acute myeloid leukemia; WBC: white blood cell count; ANC: absolute neutrophil count; PLT: platelet count; t: translocation; inv: inversion; t(8;21): AML with t(8;21)(q22;q22) RUNX1-RUNX1T1; t(15;17): APL with t(15;17)(q22;q12) PML-RARA; inv(16): AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22) CBFB-MYH11; WT: wild-type; NPM1: nucleophosmin 1; FLT3-ITD: FMS-like tyrosine kinase 3 internal tandem duplication; standard “3+7”: standard induction chemotherapy with anthracyclines and cytarabine; MEC: mitoxantrone, etoposide and intermediate-dose Ara-C; NOVE: mitoxantrone and etoposide; HiDAC: high-dose Ara-C; ID-ARAC: idarubicin and Ara-C; DN-ARAC: daunorubicin and Ara-C; PRBC: packed red blood cells
| References | ▴Top |
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099-2107.
doi pubmed - Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474.
doi pubmed - Dollinger M. Guidelines for Hospitalization for Chemotherapy. Oncologist. 1996;1(1 & 2):107-111.
pubmed - Leger C, Sabloff M, McDiarmid S, Bence-Bruckler I, Atkins H, Bredeson C, Zhang H, et al. Outpatient autologous hematopoietic stem cell transplantation for patients with relapsed follicular lymphoma. Ann Hematol. 2006;85(10):723-729.
doi pubmed - Bredeson C, Perry G, Martens C, McDiarmid S, Bence-Bruckler I, Atkins H, Serna D, et al. Outpatient total body irradiation as a component of a comprehensive outpatient transplant program. Bone Marrow Transplant. 2002;29(8):667-671.
doi pubmed - Summers N, Dawe U, Stewart DA. A comparison of inpatient and outpatient ASCT. Bone Marrow Transplant. 2000;26(4):389-395.
doi pubmed - Allan DS, Buckstein R, Imrie KR. Outpatient supportive care following chemotherapy for acute myeloblastic leukemia. Leuk Lymphoma. 2001;42(3):339-346.
doi pubmed - Savoie ML, Nevil TJ, Song KW, Forrest DL, Hogge DE, Nantel SH, Shepherd JD, et al. Shifting to outpatient management of acute myeloid leukemia: a prospective experience. Ann Oncol. 2006;17(5):763-768.
doi pubmed - Moller T, Nielsen OJ, Welinder P, Dunweber A, Hjerming M, Moser C, Kjeldsen L. Safe and feasible outpatient treatment following induction and consolidation chemotherapy for patients with acute leukaemia. Eur J Haematol. 2010;84(4):316-322.
doi pubmed - Eisele L, Gunther F, Ebeling P, Nabring J, Duhrsen U, Durig J. Outpatient management of acute myeloid leukemia after intensive consolidation chemotherapy is feasible and reduces hospital treatment costs. Onkologie. 2010;33(12):658-664.
doi pubmed - Hamadah A, Schreiber Y, Toye B, McDiarmid S, Huebsch L, Bredeson C, Tay J. The use of intravenous antibiotics at the onset of neutropenia in patients receiving outpatient-based hematopoietic stem cell transplants. PLoS One. 2012;7(9):e46220.
doi pubmed - Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919.
doi pubmed - Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694.
doi pubmed - Canadian Cancer Society, Statistics Canada, Public Health Agency of Canada. Canadian Cancer Statistics 2014. 2014.
- Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119(1):34-43.
doi pubmed - Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684-1692.
doi pubmed - Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724-3734.
doi pubmed - Savic A, Kvrgic V, Rajic N, Urosevic I, Kovacevic D, Percic I, Popovic S. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leuk Res. 2012;36(4):479-482.
doi pubmed - Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624-627.
doi pubmed - Nissim R, Rodin G, Schimmer A, Minden M, Rydall A, Yuen D, Mischitelle A, et al. Finding new bearings: a qualitative study on the transition from inpatient to ambulatory care of patients with acute myeloid leukemia. Support Care Cancer. 2014;22(9):2435-2443.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


