| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 5, Number 1, March 2016, pages 30-33
A Novel Approach for Treatment of Cold Agglutinin Syndrome-Related Severe Hemolysis
Anteneh Tesfayea, b, Catherine Broomea
aLombardi Comprehensive Cancer Center, Medstar Georgetown University Hospital, 3800 Reservoir Rd. NW, Washington DC 20007, USA
bCorresponding Author: Anteneh Tesfaye, Lombardi Comprehensive Cancer Center, Medstar Georgetown University Hospital, 3800 Reservoir Rd. NW, Washington DC 20007, USA
Manuscript accepted for publication January 20, 2016
Short title: Cold Agglutinin Syndrome-Related Hemolysis
doi: http://dx.doi.org/10.14740/jh242w
| Abstract | ▴Top |
Intravascular hemolysis related to cold agglutinin syndrome results from the activation of the classical complement pathway by red blood cell (RBC) surface I/i antigen bound autoantibodies. Despite built-in mechanisms that limit continued downstream complement activation, some patients may develop life-threatening intravascular hemolysis due to the formation of membrane attack complexes. We present a case of severe intravascular hemolysis due to cold agglutinin syndrome that was treated successfully using proximal complement inhibition with commercial C1 esterase inhibitor. The role of complement inhibition in controlling the intravascular hemolysis and resolving the immune dysfunction that leads to the syndrome is discussed.
Keywords: Cold agglutinin syndrome; Intravascular hemolytic anemia; Complement pathway; Complement inhibition; C1 esterase inhibitor
| Introduction | ▴Top |
Hemolysis related to cold agglutinin syndrome is the result of red blood cell (RBC) surface I/i antigen bound antibodies activating the classical complement pathway. This culminates in the deposition of C3b on the surface of RBCs. The RBCs are subsequently cleared by the reticuloendothelial system, the primary mechanism of the low-grade chronic hemolysis observed in most patients [1, 2].
RBC membrane-bound CD55 and CD59 normally limit downstream complement activation. In some patients, especially during heightened immune stimulation, complement activation may proceed beyond C3b formation, leading to formation of C5a and C5b-9 (the membrane attack complex) and intravascular hemolysis which can be severe and life-threatening [1, 2].
We report a case of cold agglutinin syndrome presenting with acute intravascular hemolysis. We hypothesized that immediate proximal inhibition of the complement cascade with a commercially available C1 esterase inhibitor would be an effective strategy to halt intravascular hemolysis.
| Case Report | ▴Top |
A 61-year-old African American male with metastatic adenocarcinoma of the lung, receiving second-line pemetrexed, presented with severe anemia 2 weeks after his last treatment. Although complete blood count (CBC) could not be performed due to autoagglutination of the anticoagulated blood specimen, even at room temperature, his hemoglobin was measured at 3.8 g/dL on blood gas analysis. Haptoglobin was undetectable and lactate dehydrogenase was 1,034 IU/L, consistent with a significant intravascular hemolysis. His ABO blood group was A+D+. Both indirect Coombs and DAT were positive for C3d (3+) and negative for IgG. His peripheral smear at room temperature revealed severe agglutination of RBCs, which improved after incubation at 37°. Cold agglutinin titers using I+ve/i-ve reagent RBCs were 1:1,024 at room temperature (22 °C), 1:1,024 at 4 °C, and 1:32 at 37 °C. With I-ve/i+ve reagent RBCs, the titers were 1:256 at 22 °C, 1:1,024 at 4 °C, and 1:1:8 at 37 °C.
Daily treatment with the commercially available C1 esterase inhibitor, Berinert®, at a dose of 20 units/kg/day intravenously, was initiated along with prednisone 1 mg/kg/day and four weekly doses of rituximab 375 mg/m2. Transfusions were avoided due to difficulty with cross-matching and concerns of additional hemolysis of transfused RBCs. CBC, haptoglobin, and LDH were evaluated prior to and 2 h after the administration of Berinert® daily. Following the administration of each dose of C1 esterase inhibitor, there was an immediate improvement in the hemolysis parameters and a modest rise in hemoglobin (Fig. 1, 2).
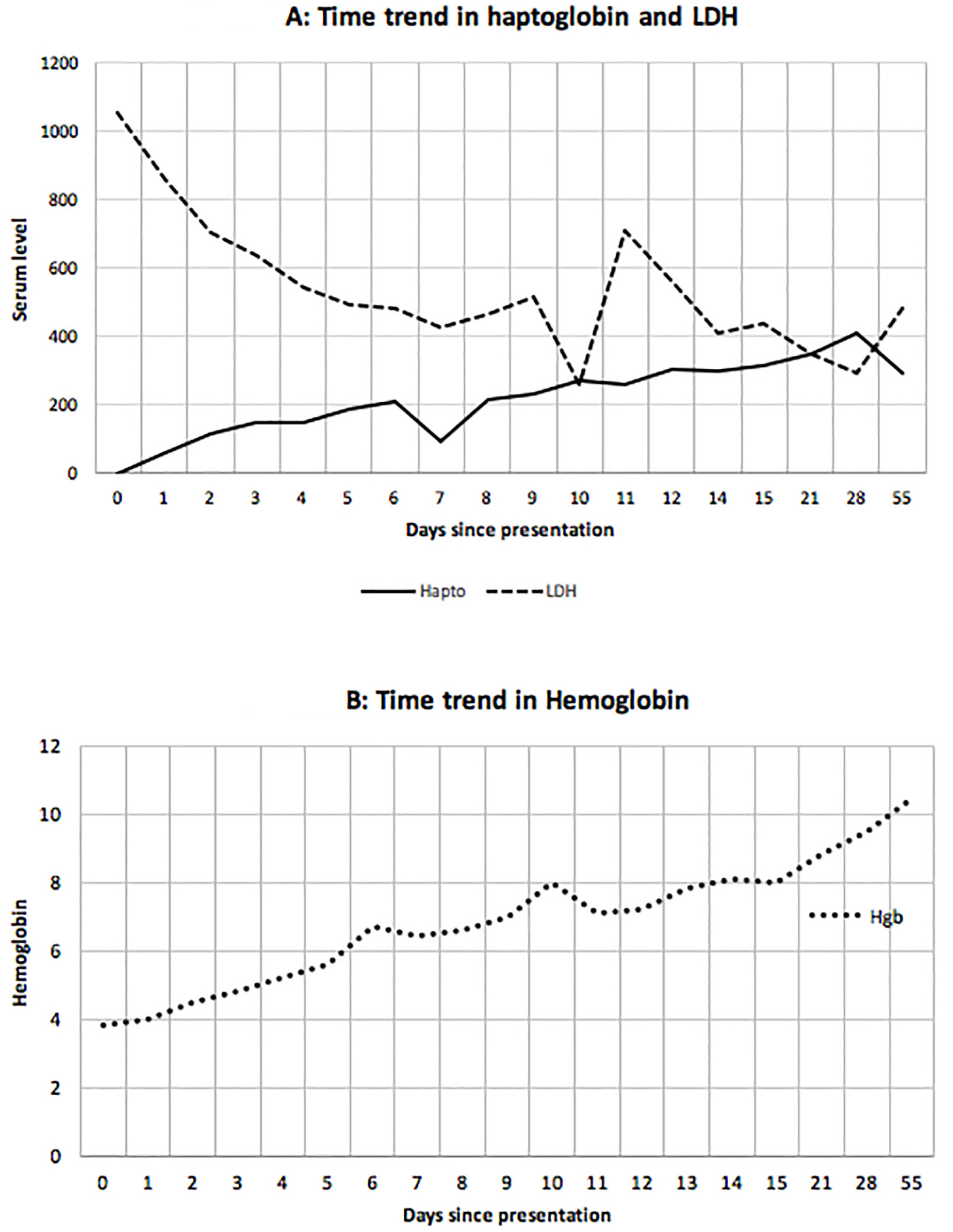 Click for large image | Figure 1. Time trend in haptoglobin and lactate dehydrogenase (LDH) (A) and hemoglobin (B). Day 0 was when the consult was received. Berinert was administered on day 0 to day 8 (except day 6). Rituximab was given on days 4 and 11. The other two doses were given as outpatient. Prednisone 1 mg/kg/day was started on day 0 and was continued till tapered off as outpatient. |
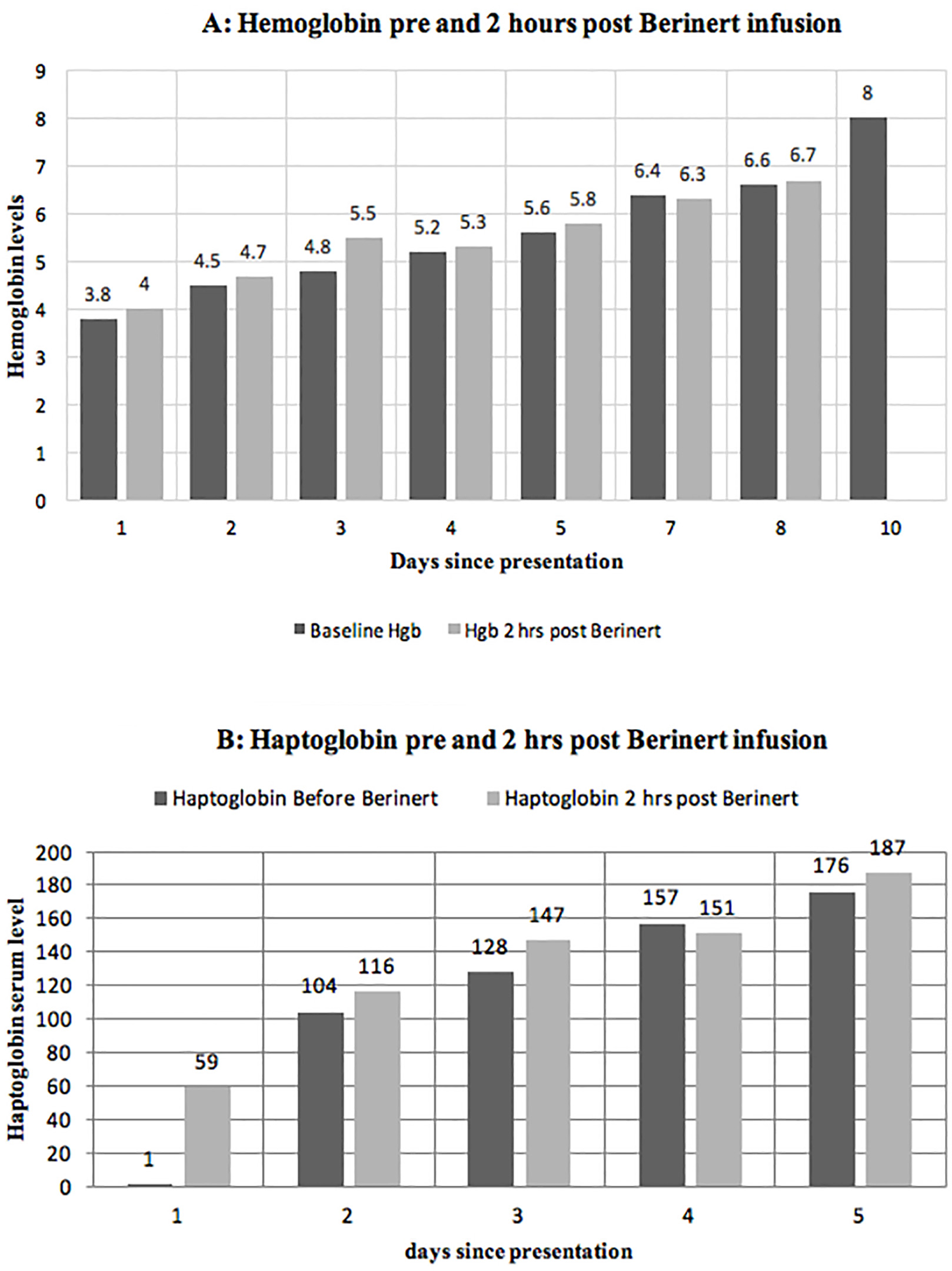 Click for large image | Figure 2. Levels of hemoglobin (A) and haptoglobin (B) before and 2 h after the administration of Berinert showing an immediate improvement in hemolysis. |
Patient’s hemoglobin increased to baseline level at day 14 after a total of eight doses of Berinert® and two doses of rituximab without any packed RBC transfusion (Fig. 1). We were able to accurately perform CBC analysis on day 7 after he received six daily doses of Berinert®, daily prednisone and one dose of rituximab. On day 14, after eight doses of Berinert®, 2 weeks of steroids, and two doses of rituximab, the cold agglutinin titers using I+ve/i-ve reagent RBCs were 1:4 at room temperature (22 °C), 1:64 at 4 °C, and 1:1 at 37 °C, indicating a significant response to treatment. The hemoglobin has continued to slowly improve with no evidence of ongoing hemolysis after completion of the planned therapy including a taper-off of prednisone.
| Discussion | ▴Top |
Patients with cold agglutinin syndrome usually present with chronic low-grade hemolytic anemia. There may be prominent autoagglutination of their red cells in vivo and/or in vitro, especially at cold temperatures. Presentation with acute, severe intravascular hemolysis is rare [1, 3].
Cold agglutinin syndrome has been linked most often to underlying lymphoproliferative disorders [4]; however, rare cases of paraneoplastic cold agglutinin syndrome associated with solid tumors have been reported [5-7]. In the absence of any other findings, we hypothesize that the cold agglutinin syndrome may be a paraneoplastic manifestation of active lung adenocarcinoma.
In in vitro models, the proximal inhibition of complement using a monoclonal antibody against C1 esterase has resulted in the inhibition of C3b formation, RBC phagocytosis, and downstream complement mediated intravascular hemolysis [2]. The use of plasma derived C1 esterase inhibitor in a patient with hemolytic crisis caused by warm antibody autoimmune hemolytic anemia has been shown to improve response to packed red cell transfusion [8].
Reports indicate rituximab may have a response rate as high as 60% in reducing cold agglutinin titers. Almost all of the responses were partial responses and there was high rate of relapse [1, 4]. Berentsen and colleagues showed that the median time to response in patients treated with riutximab was 1.5 months. The median duration of response observed was 11 months [4]. The utility of steroids in treating cold agglutinin syndrome is reported to be very limited [1, 3, 4].
There is a growing body of evidence indicating an integral role of the complement system in immune regulation, i.e. antibody production by B lymphocytes, activity of effector T cells and activity of regulatory T cells.
The binding of complement protein fragment C3dg to the B-cell complement receptor, (CR2, CD21) facilitates antibody production by B cells. It has been demonstrated that complement depletion leads to impaired antibody production [9, 10].
Downstream complement activation products have been implicated in the generation of effector T cells that help maintain immune response by enhancing T-cell proliferation and diminishing T-cell apoptosis [11]. The complement proteins have also been shown to function as paracrine and autocrine stimulators of effecter T cells through their G protein coupled receptors. Disabling this interaction resulted in diminished effector T-cell responses [11]. T cells deficient in complement interaction undergo accelerated cell death [12].
Regulatory T cells play an indispensable role in maintaining immunologic unresponsiveness to self-antigens and in suppressing excessive immune responses deleterious to the host [13]. Regulatory T-cell generation, stability, and suppressive function are decreased by C3a and C5a signaling [14]. The binding of C3a and C5a to receptors on regulatory T cells leads to uninhibited autoreactivity and possibly to immune-related organ injury and transplant organ rejection [15].
There has been speculation that inhibition of the complement cascade may help suppress the activation of antibody production and cell-mediated immunity, including in the post-transplant setting [10, 15, 16].
Berinert® is a plasma-derived parenteral C1 esterase inhibitor concentrate that is commercially available in the United States for the treatment of hereditary angioedema. When used for the treatment of hereditary angioedema, the drug is well tolerated with few adverse effects. A possible adverse effect of this therapy is an increased risk of venous thromboembolism, although this has not been demonstrated consistently [17].
Utilizing a novel approach of proximal complement blockade with a C1 esterase inhibitor, we successfully treated a life-threatening autoimmune hemolytic crisis associated with cold agglutinin syndrome. In addition, we hypothesize that the use of proximal complement inhibition may have augmented the resolution of antibody production by dampening B-cell activity and enhancing regulatory T-cell function.
Acknowledgement
Drs. Tesfaye and Broome treated the patient and co-authored the short report. The authors thank Dr. Gerald Sandler for conducting and interpreting the serologic studies.
| References | ▴Top |
- Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26(3):107-115.
doi pubmed - Shi J, Rose EL, Singh A, Hussain S, Stagliano NE, Parry GC, Panicker S. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123(26):4015-4022.
doi pubmed - Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15.
doi pubmed - Berentsen S, Ulvestad E, Gjertsen BT, Hjorth-Hansen H, Langholm R, Knutsen H, Ghanima W, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103(8):2925-2928.
doi pubmed - Wortman J, Rosse W, Logue G. Cold agglutinin autoimmune hemolytic anemia in nonhematologic malignancies. Am J Hematol. 1979;6(3):275-283.
doi pubmed - Cao L, Kaiser P, Gustin D, Hoffman R, Feldman L. Cold agglutinin disease in a patient with uterine sarcoma. Am J Med Sci. 2000;320(5):352-354.
doi pubmed - Al-Matham K, Alabed I, Zaidi SZ, Qushmaq KA. Cold agglutinin disease in fibrolamellar hepatocellular carcinoma: a rare association with a rare cancer variant. Ann Saudi Med. 2011;31(2):197-200.
doi pubmed - Wouters D, Stephan F, Strengers P, de Haas M, Brouwer C, Hagenbeek A, van Oers MH, et al. C1-esterase inhibitor concentrate rescues erythrocytes from complement-mediated destruction in autoimmune hemolytic anemia. Blood. 2013;121(7):1242-1244.
doi pubmed - Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140(1):126-145.
doi pubmed - Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348-350.
doi pubmed - Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759-1766.
doi pubmed - Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425-435.
doi pubmed - Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775-787.
doi pubmed - Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210(2):257-268.
doi pubmed - Mathern DR, Heeger PS. Molecules Great and Small: The Complement System. Clin J Am Soc Nephrol. 2015;10(9):1636-1650.
doi pubmed - van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol. 2013;190(12):5921-5925.
doi pubmed - Busse P, Bygum A, Edelman J, Lumry W, Machnig T, Martinez-Saguer I, Rojavin M. Safety of C1-esterase inhibitor in acute and prophylactic therapy of hereditary angioedema: findings from the ongoing international Berinert patient registry. J Allergy Clin Immunol Pract. 2015;3(2):213-219.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


