| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 5, Number 1, March 2016, pages 17-24
Quantitative Expression of Toll-Like Receptors TLR-7 and TLR-9 on Peripheral Blood Mononuclear Cells in Leukemias
Mona G. Morsia, Maha M. El Gharabawya, Nahla A. Hamedb, Mohamed M. El Sawyc, Noha M. Abou Seadaa, d, Rola A. Hashada
aMicrobiology & Immunology, Faculty of Medicine, Alexandria University, Egypt
bInternal Medicine Hematology Unit, Faculty of Medicine, Alexandria University, Egypt
cClinical and Chemical Pathology, Faculty of Medicine, Alexandria University, Egypt
dCorresponding Author: Noha M. Abou Seada, Microbiology & Immunology, Faculty of Medicine, Alexandria University, Egypt
Manuscript accepted for publication February 29, 2016
Short title: Expression of TLR-7 and 9 in Leukemias
doi: http://dx.doi.org/10.14740/jh249w
| Abstract | ▴Top |
Background: Toll-like receptors (TLRs) are agents of innate and adaptive immunity, involved in tumor activation. The expression and functionality of TLR on leukemic cells have seldom been investigated. The aims of the study were to throw some light on the quantitative expression of TLR-7 and TLR-9 on leukemic cells and to compare it with their expression on mononuclear cells of healthy volunteers.
Methods: Ninety subjects were included: 30 acute leukemia, 30 chronic leukemia, and 30 healthy controls. Peripheral blood mononuclear cells (PBMCs) were isolated, and the expressions of TLR-7 and 9 were detected by real-time PCR.
Results: Expression of TLR-7 in acute leukemia was significantly higher than normal controls, while TLR-9 did not show significant difference. TLR-7 and TLR-9 expression in chronic myeloid leukemia (CML) was significantly lower than normal controls. Expression of TLR-7 and TLR-9 in chronic lymphocytic leukemia (CLL) was significantly higher than normal controls.
Conclusion: Although TLR-7 and TLR-9 could be expressed in acute leukemia cells, only TLR-7 shows significantly different expression with controls, indicating that TLR-7 may play a role in the immune escape from acute leukemia which suggests a potential role for TLR-7 agonists in immunotherapy. The mRNA expressions of TLR-7 and TLR-9 are significantly reduced in CML patients, which may be the main reason of function defects of PBMCs. The mRNA expressions of TLR-7 and TLR-9 are significantly increased in CLL. This might prove relevant for elucidating the immune mechanisms underlying CLL and define subgroups of patients who might benefit from treatment with specific TLR-7 and TLR-9 ligands.
Keywords: Acute myeloid leukemia; Acute lymphoblastic leukemia; Chronic myeloid leukemia; Chronic lymphocytic leukemia; Toll-like receptor 7; Toll-like receptor 9
| Introduction | ▴Top |
Toll-like receptors (TLRs), expressed predominantly on antigen-presenting cells, belong to the family of pattern-recognition receptors (PRRs). In humans, TLR family consists of 10 members (TLRs 1-10) [1]. It plays an essential role in immune response against microbial pathogens by recognizing specific microbial molecular components. After being activated, TLRs initiate a signaling cascade resulting in the stimulation of innate and adaptive immune responses targeting the invading pathogen [2]. Although TLRs have been implicated as the first line defense in human for anti-microbial responses, they also participate in the pathophysiology of many inflammatory and immune diseases, including cancer [3, 4].
TLRs 7 and 9 are members of the Toll-like family of receptors, and sense infection by detecting molecular structures of invading microbial pathogens and initiate innate immune responses [5]. These receptors mediate adaptive immune responses by activating immune cells, such as dendritic cells (DCs). TLR7 and TLR9 are present on both immune and non-immune cells [5, 6].
Leukemia is a disease which is in part characterized by an impaired immune system, and it is probable that dysregulation of TLRs which play an important role in the preservation of a physiologic immune system would be observed in leukemic states. Leukemia is a broad classification of malignancy of the blood forming elements which upon further distinction of the lineage and differentiation of the leukemic clone encompasses a rather vast number of specific leukemic subtypes [7]. For general categorization purposes, the types of leukemia are often divided into acute and chronic cases as well as being distinguished by the myeloid or lymphocytic nature of the malignant clonal population. In states of leukemic burden, contributing effector molecules of the immune system would be dysregulated and thus found to be expressed at levels which differed from their normal expression characteristics in the setting of a properly functioning immune system. TLR7 and TLR9 were the individual TLRs evaluated in this investigation [8].
Acute myeloid leukemia (AML) is a common form of acute leukemia and remains a difficult disease with poor survival in patients who have failed standard therapy. New therapeutic strategies are needed to achieve longer survival and improve cure rates in AML patients [9]. The expression and functionality of TLR on AML cells have seldom been investigated. However, such structures could provide an interesting therapeutic target if they are functional and their triggering induces relevant modifications of tumor cells [9, 10].
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and although current therapy is widely effective, relapse remains a significant clinical problem for which new treatment strategies are required. The ligation of TLR on antigen-presenting cells stimulates the generation of strong T-cell helper type 1 (Th1) adaptive immune responses. Although TLR-9 ligation has been shown to enhance immunogenicity of a number of leukemia cell types, there have been few reports of the effects mediated through other TLRs [11]. In ALL cell lines, TLR-1, TLR-2, TLR-3, TLR-4, TLR-6 and TLR-7 are expressed albeit at variable levels. In bone marrow of ALL patients with > 90% blasts TLR-2 mRNA can be detected in the majority of the samples [12].
TLRs may play a role in chronic hematologic malignancies, especially in chronic lymphocytic leukemia (CLL). The expression of TLR in CLL is quite heterogeneous between patients but most cases express TLR-1, TLR-2, TLR-6 and TLR-10 on the cell surface, and TLR-7, TLR-8, TLR-9 within endosomes, thus resembling normal mature B lymphocytes [13]. The full expression profile of mRNA for TLR and signaling molecules was studied in a large group of CLL patients to search for potential differences in specific subsets of patients [14, 15]. TLR-1, TLR-2, TLR-6, TLR-7, TLR-9 and TLR-10 were expressed in CLL, while the other TLRs were low or negative. As for TLR-4 and TL-R8, a significant variation was observed among different samples [16].
The objectives were to detect and quantitate the expression of TLR-7 and TLR-9 on leukemic cells and to compare it with their expression on mononuclear cells of healthy volunteers.
| Materials and Methods | ▴Top |
Ninety subjects were included in the study, and divided into three groups: group I: 30 acute leukemia cases (including 15 AML and 15 ALL); group II: 30 chronic leukemia cases (including 15 chronic myeloid leukemia (CML) and 15 CLL); and group III: 30 healthy controls. All patients enrolled in this study were selected from Hematology Unit of Internal Medicine Department, Alexandria Main University Hospital.
Informed consent was obtained from each patient and the study protocol was approved by the Ethics Committee of the Faculty of Medicine, Alexandria University.
Peripheral blood mononuclear cells (PBMCs) isolation
Peripheral blood samples (3 - 5 mL) were collected in EDTA vacutainer tubes from both patients and controls, and PBMCs were isolated using the Ficoll HypaqueTM density-gradient centrifugation technique (Sigma). Cells were washed three times in RPMI 1640 complete medium by centrifugation for 10 min at 400× g. The supernatant was aspirated and the cell pellet was tapped and resuspended in a known volume of complete RPMI medium supplemented with 10% fetal bovine serum (FBS). Cell pellets were used for RNA isolation.
RNA isolation, reverse transcription, and quantitative real-time PCR (qPCR)
Total RNA was extracted using Qiagen RNeasy Isolation Kit (QIAGEN, Hilden, Germany) from 1 × 106 - 2 × 106 PBMCs. Absorbance was measured at 260 and 280 nm for measuring RNA concentration as well as protein contamination factor.
Reverse transcription and real-time PCR were performed with the SensiFASTTM SYBR No-ROX One-Step Kit (Bioline Ltd, UK) using an Applied Biosystems StepOneTM Real-Time PCR (Applied Biosystems, Foster, CA, USA).
Gene specific primers for human TLR-7 and TLR-9 and GAPDH genes were selected [17-19]. The qPCR reactions were performed in a reaction volume of 20 μL, with 10 μL 2× SensiFASTTM SYBR No-ROX One-Step Mix, 400 nM of each primer, 0.2 μL reverse transcriptase, 0.4 μL RiboSafe RNase inhibitor and 2 μL template. The reaction conditions were as follows: 45 °C for 10 min (reverse transcription), 95 °C for 2 min, followed by 40 cycles at 95 °C for 5 s, 60 °C for 10 s and 72 °C for 5 s. Fluorescence was measured during the 72 °C step for each cycle. The relative quantitation of target gene expression was calculated by comparative Ct method. To normalize the amount of sample cDNA, one endogenous control transcript of housekeeping gene coding for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used.
To control for specificity of the amplification products, a melting curve analysis was performed. No amplification of unspecific products was observed.
Statistical analysis
Data were fed to the computer using IBM SPSS software package version 20.0.
Qualitative data were described using number and percent. Comparison between different groups regarding categorical variables was tested using Chi-square test. Quantitative data were described using mean and standard deviation for normally distributed data while abnormally distributed data were expressed using median, minimum and maximum. Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level.
| Results | ▴Top |
The study was conducted on 90 subjects, and divided into three groups: group I: 30 acute leukemia cases (including 15 AML and 15 ALL); group II: 30 chronic leukemia cases (including 15 CML and 15 CLL); and group III: 30 healthy controls.
Analysis of expression level of TLR-7 and TLR-9 mRNA in PBMC by real-time quantitative RT-PCR
To quantify the expression level of TLR-7 and TLR-9 mRNA in PBMC of healthy controls and leukemia patients, we used real-time quantitative RT-PCR. Accordingly, the expression of TLR-7 in AML cells (1.44 ± 0.126) was significantly higher than the normal controls (1.023 ± 0.061) (P < 0.05), while the expression of TLR-9 (1.35 ± 0.187) did not show significant difference with normal controls (1.245 ± 0.112) (P > 0.05).
Similarly, the expression of TLR-7 in ALL cells (1.447 ± 0.1611) was significantly higher than the normal controls (1.023 ± 0.061) (P < 0.05), while the expression of TLR-9 (1.162 ± 0.191) did not show significant difference with normal controls (1.245 ± 0.112) (P > 0.05).
Meanwhile, the expression of TLR-7 in CLL cells (0.341 ± 0.16) was significantly lower than the normal controls (1.023 ± 0.061) (P < 0.05) and the expression of TLR-9 (0.442 ± 0.161) also was significantly lower than the normal controls (1.245 ± 0.112) (P < 0.05).
On the other hand, the expression of TLR-7 in CLL cells (1.462 ± 0.07) was significantly higher than the normal controls (1.023 ± 0.061) (P < 0.05) and the expression of TLR-9 (1.425 ± 0.11) was also significantly higher than the normal controls (1.245 ± 0.112) (P < 0.05) (Tables 1, 2, Figs. 1-4).
 Click to view | Table 1. Comparison Between Peripheral Blood Expression of TLR-7/GAPDH mRNA in PBMCs in Different Studied Groups and Controls |
 Click to view | Table 2. Comparison Between Peripheral Blood Expression of TLR-9/GAPDH mRNA in PBMCs in Different Studied Groups and Controls |
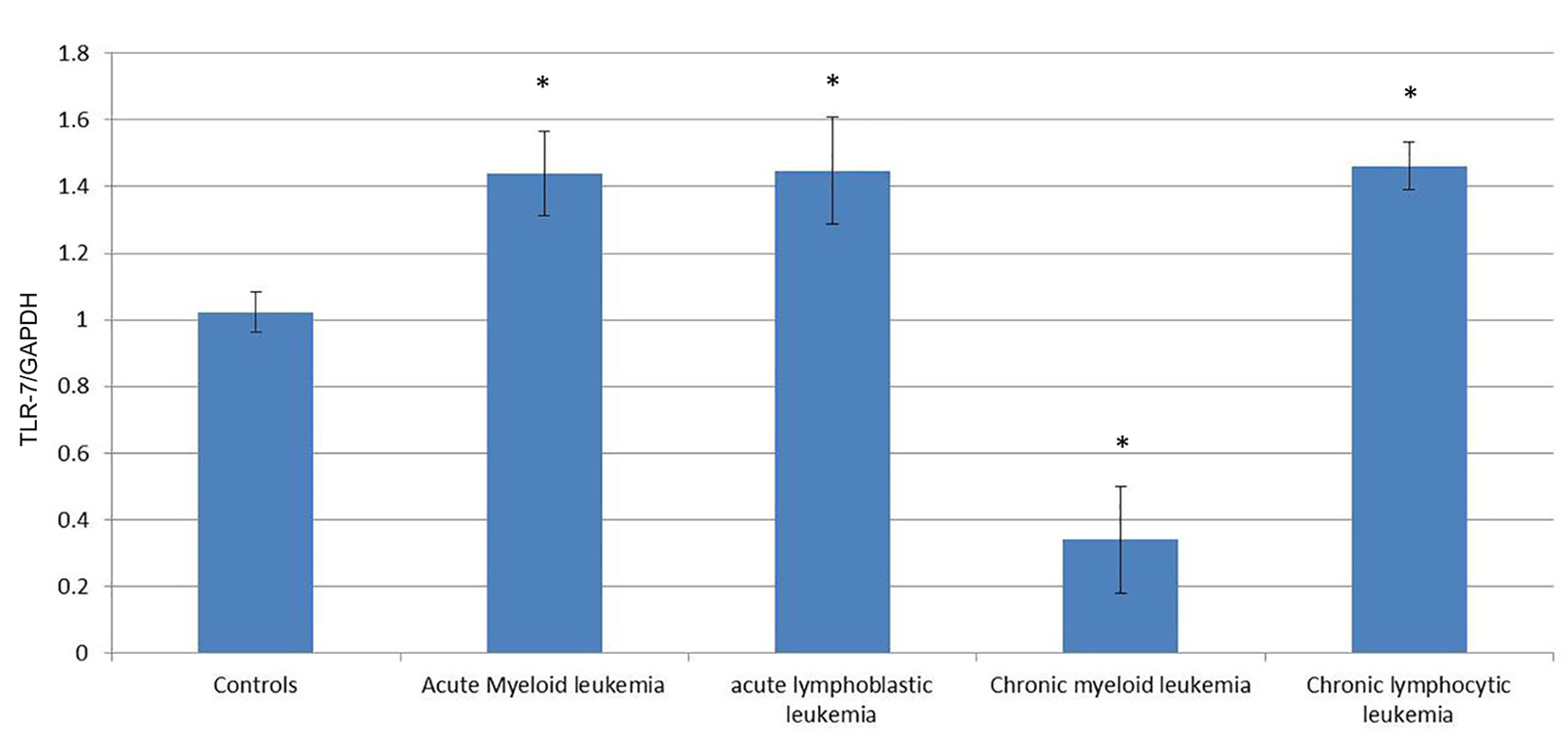 Click for large image | Figure 1. Comparison of the expression of TLR-7/GAPDH mRNA in PBMCs between different groups. |
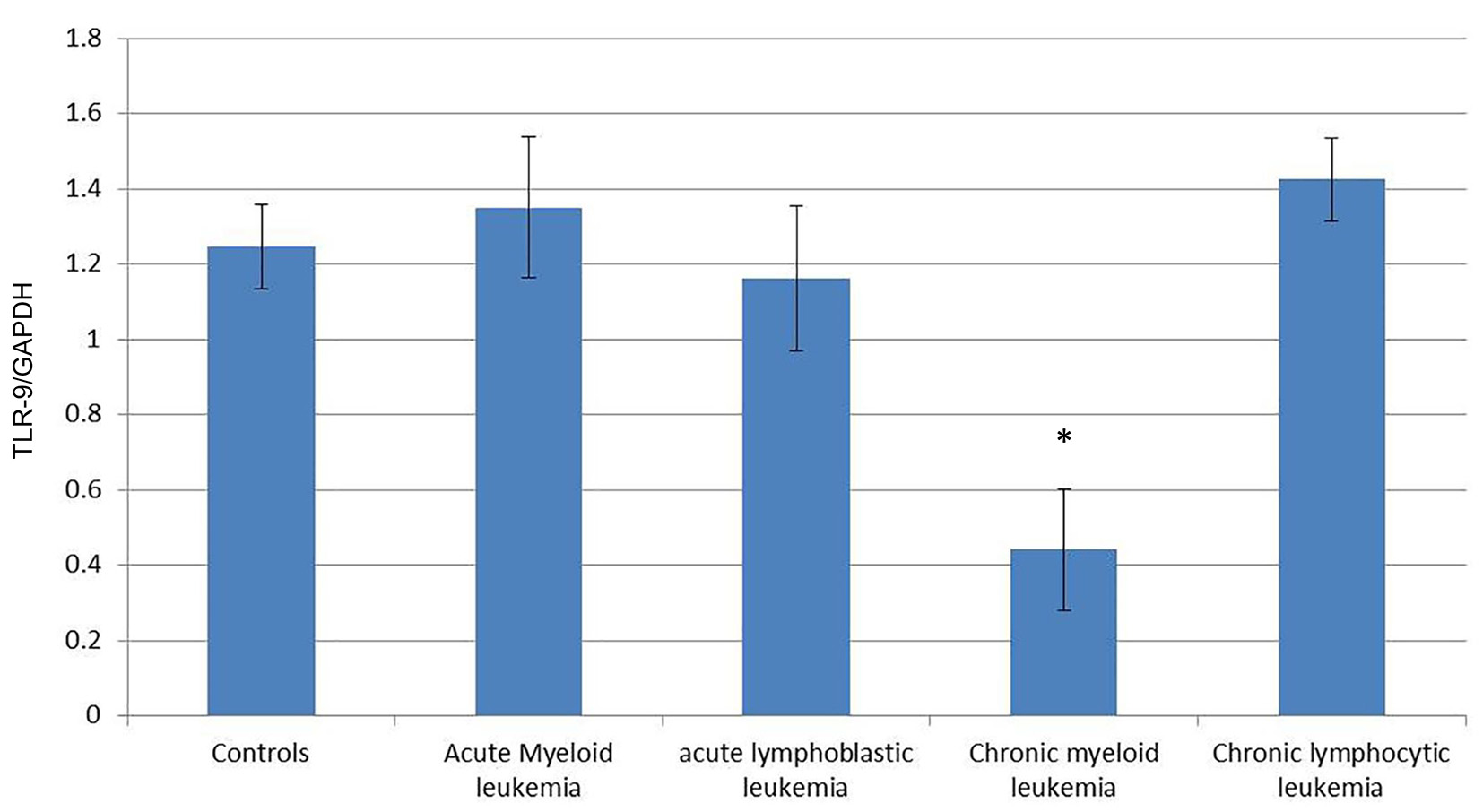 Click for large image | Figure 2. Comparison of the expression of TLR-9/GAPDH mRNA in PBMCs between different groups. |
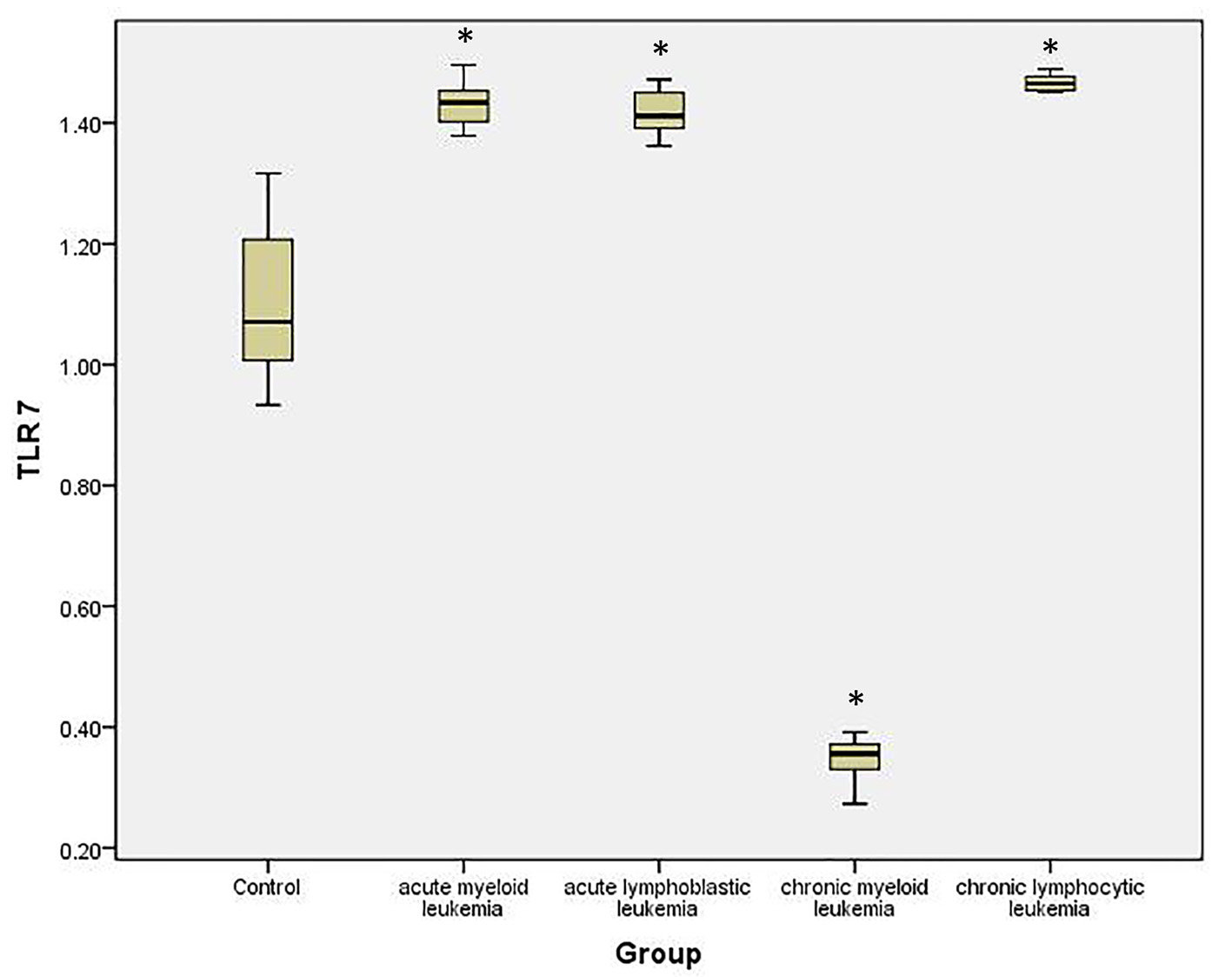 Click for large image | Figure 3. Relative expression of TLR-7 by RT-PCR data in different studied groups and controls. |
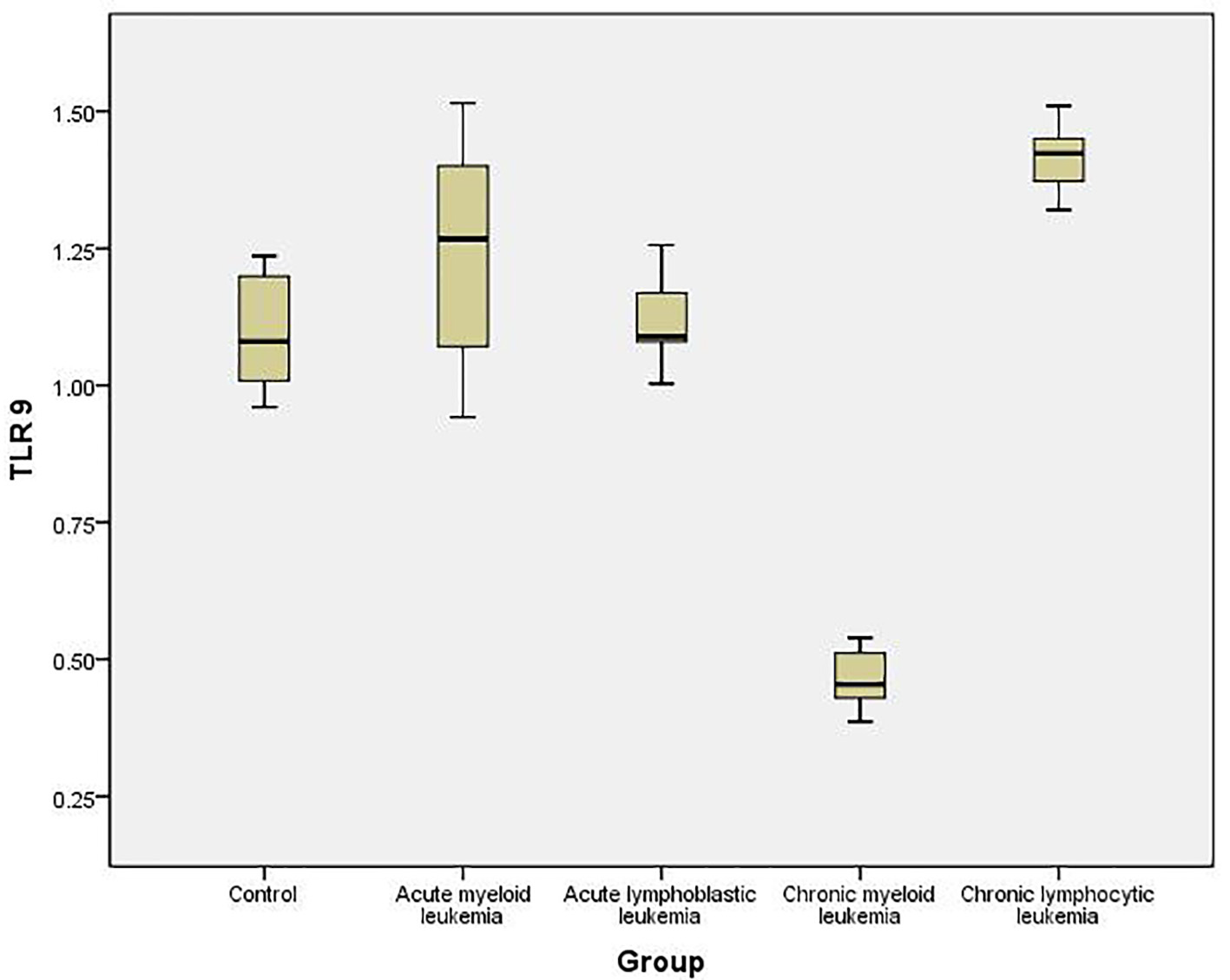 Click for large image | Figure 4. Relative expression of TLR-9 by RT-PCR data in different studied groups and controls. |
To examine the relationship between TLR-7 and TLR-9 in leukemias, the correlation coefficient between TLR-7 and TLR-9 was studied and demonstrated no correlation between both of them in AML (r = 0.104; P > 0.05) and ALL (r = 0.211; P > 0.05). In contrast to acute leukemias, a significant mutual correlation was seen to exist between TLR-7 and TLR-9 in CML (r = 0.336; P < 0.05) and CLL (r = 0.308; P < 0.05).
| Discussion | ▴Top |
TLRs play an important role in the host defense against microorganisms. TLRs are mainly expressed in human immune-related cells, such as monocytes, neutrophils, macrophages, dendritic cells, T cells, B cells and NK cells. The expression or up-regulation of TLRs has been demonstrated in some tumors and tumor cell lines but the role of TLRs in pathogenesis and development of leukemias remains unclear [10].
Our data demonstrated a statistically significant difference between the mean level of TLR-7/GAPDH mRNA expression in AML patients and that in normal cases. Meanwhile, there was no statistically significant difference between these two groups regarding the level of TLR-9/GAPDH mRNA expression. Our findings were similar to those of previous studies [8, 20, 21] which evaluated the quantitative expression of TLRs in patients with newly diagnosed or relapsed AML and concluded that the highest level of TLR expression was seen for TLR-7 while there was no significant difference in the expression of the other TLRs compared to the healthy volunteers. Similarly, other studies [6, 22, 23] demonstrated that the level of TLR-7/β-actin mRNA expression was significantly elevated in AML patients compared to normal cases (P < 0.05). Meanwhile there was no statistically significant difference between the level of TLR-9/β-actin mRNA expression in AML patients and in normal cases (P > 0.05).
Similarly in ALL, there was a statistically significant difference between the mean level of TLR-7/GAPDH mRNA expression in patients and normal cases. While, there was no significantly statistic difference between these two groups as regards the level of TLR-9/GAPDH mRNA expression. These findings clearly agree with the results of Corthals et al (2005) [11] who tested the expression of TLRs 1-7 in ALL cell lines and found that each of the TLRs was detected in several cell lines, although the level of expression varied considerably between the lines. TLR-1 was the only receptor expressed at high levels in all cell lines. This result showed that mRNA for TLRs 1-7 were present in a number of BCP-ALL cell lines, and suggested that these cell lines may be responsive to the respective TLR ligands. Liang et al (2008) [24] stated that human B-ALL cell lines and primary B-ALL cells express high levels of TLR-7 mRNA and proteins. Also, Isaza-Correa et al (2014) [12] reported that in ALL cell lines, TLR-1, TLR-2, TLR-3, TLR-4, TLR-6 and TLR-7 are expressed albeit at variable levels. Although Bourke et al (2003) [15] reported that normal and malignant B cells show a distinct TLR mRNA expression profile, which includes particularly high levels of TLR-9 and TLR-10, a limited study by Reid et al (2005) suggested a lack of TLR-9 expression in pre-B-ALL cell lines [25].
Thus, our data and those from literature strongly demonstrate that TLR-7 and TLR-9 could be expressed in acute leukemia cells (myeloid and lymphoblastic), but that only TLR-7 shows a significantly higher expression level compared to controls, supporting that TLR-7 may play a role in the immune escape from acute leukemias (AML and ALL). These findings suggest that TLR-7 targeting may inhibit the growth and induce apoptosis of leukemic cells, providing new insights into the biology and therapy of acute leukemias.
On the other hand, in this study, mRNA expression of TLR-7 and TLR-9 was significantly reduced in CML patients than in normal cases. Few data are available on mRNA expressions of TLRs in CML patients. However, Xu et al (2012) stated that impaired IFN-α production from pDC may contribute to the immunopathogenesis of chronic HBV infection, which may be the result of a reduced amount of pDCs as well as decreased expression of TLR-7 and TLR-9 on pDCs [26].
Thus, our current study and those cited may reflect that the significant reduction of mRNA expressions of TLR-7 and TLR-9 may be the main reason of function defects of PBMCs indicating that TLR-7 and TLR-9 may be involved in the pathogenesis of CML.
On the contrary to CML, the mRNA expression of TLR-7 and TLR-9 was significantly higher in CML patients than in normal cases. These results were similar to those of Spaner et al (2007) [27] who stated that CLL might be especially amenable to TLR agonists because it was an immunologically susceptible tumor with strong expression of several TLRs, particularly TLR-7 and TLR-9. TLR agonists may indirectly clear CLL cells by enhancing the activity of natural killer and tumor-reactive T cells, or by altering the tumor microenvironment and inhibiting angiogenesis. Liang et al (2010) [28] proved that human B-CLL cells express high levels of TLR-9 and can be potently activated by CpG-B ODNs. Arvaniti (2011) [16] reported that the data available on TLR expression in CLL were limited but have essentially shown that TLR-7 and TLR-9 were virtually always expressed. In addition to TLR-7 and TLR-9, CLL cells can also express TLR-1, TLR-2, TLR-6 and TLR-10. Meanwhile, Muzio et al (2012) [29] have studied the full expression profile of mRNA for TLR and signaling molecules in a large group of CLL patients to search for potential differences in specific subsets of patients. CLL cells showed high expression of TLR-7, intermediate expression of TLR-1, TLR-6 and TLR-10 and low expression of TLR-2, TLR-4, TLR-8 and TLR-9 and confirmed that CLL cells express functional TLR-9, similar to normal B lymphocytes. On the other hand, suppression of TLRs has not been studied in CLL so far, and the rationale for this may be increasing evidence of the supportive role of TLR signaling in CLL. In another study, Barcellini et al (2014) [30] stated that TLR-4 gene expression was decreased and TLR-9 was increased in CLL patients versus controls.
Thus, our present study provides evidence to indicate that these distinct patterns of TLR functional activity in cells from CLL might prove relevant for elucidating the immune mechanisms underlying the natural history of CLL and define subgroups of patients who might benefit from treatment with specific TLR-7 and TLR-9 ligands.
To examine the relationship between these two critical immunomodulatory receptors in leukemias, the correlation coefficient between TLR-7 and TLR-9 was studied and demonstrated no correlation between both of them in AML and ALL. Our results were similar to those of Klonowska-Szymczy et al (2014) [31] who stated that a significant positive correlation was recorded between TLR-3 and TLR-9 expression in PBMCs in patients with SLE but not with TLR-7.
Unfortunately, no publication regarding mutual TLR relations can be found. However, it is probable that the non-significant correlation of TLR-7 and TLR-9 stems from the higher lability of ssRNA (the ligand for TLR-7), which undergoes rapid degradation by ribonucleases and is quickly removed from circulation.
In contrast to acute leukemias, a significant positive correlation was seen to exist between TLR-7 and TLR-9 in CML and CLL. From these observations, we can conclude that an imbalance in TLR-7 and TLR-9 axis may be relevant in the pathophysiology of chronic leukemias. The imbalance in production of the two critical immunomodulatory receptors such that TLR-7 and TLR-9 is under expressed in CML and overexpressed in CLL may be associated with immunological alterations in chronic leukemias. Although it is not entirely clear what tips the balance between these two TLRs, the potential role of TLR-7 and TLR-9 and the interaction between them in leukemic patients require further investigations.
Our data agreed with another study carried by Nickerson et al (2015) [32] who reported that it is possible that TLR-7 and TLR-9 are operating in series, with signals mediated by one influencing the other either directly or indirectly postulating a direct interaction, with TLR-9 regulating signals via TLR-7 within the same cell.
Acknowledgement
We are indebted to the Faculty of Medicine, Alexandria University where this work was conducted.
Author Contributions
Prof. M. Morsi and Prof. M. Elgharabawy helped in the study design and manuscript review, Prof. N. Hamed provided the clinical guidance, sample choice and collection, and Dr. M. Elsawy, Dr. N. Abouseada and Dr. R. Hashad performed the lab work, statistical analysis and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675-680.
doi pubmed - Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250-265.
doi pubmed - Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7(10):1271-1285.
doi pubmed - El-Omar EM, Ng MT, Hold GL. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008;27(2):244-252.
doi pubmed - Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373-384.
doi pubmed - Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res. 2012;35(8):1297-1316.
doi pubmed - Jaffe ES HN, Stein H, Vardiman JW. Pathology and Genetics of Tumors of Hematopoietic and Lymphoid Tissues. Lyon 2001 Contract No.: Document Number.
- Webb RN, Cruse JM, Lewis RE. Differential cytokine and Toll-like receptor expression in leukemia. Exp Mol Pathol. 2007;83(3):464-470.
doi pubmed - Shindo M LX, Wang Z, Miller J. Toll like receptor agonists induce immunogeneicity and apoptosis of acute myeloid leukemia cells. Blood (ASH Annual Meeting Abstracts). 2007. 2007. p. Abstract 160.
- Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27(2):218-224.
doi pubmed - Corthals SL, Wynne K, She K, Shimizu H, Curman D, Garbutt K, Reid GS. Differential immune effects mediated by Toll-like receptors stimulation in precursor B-cell acute lymphoblastic leukaemia. Br J Haematol. 2006;132(4):452-458.
pubmed - Isaza-Correa JM, Liang Z, van den Berg A, Diepstra A, Visser L. Toll-like receptors in the pathogenesis of human B cell malignancies. J Hematol Oncol. 2014;7:57.
doi pubmed - Muzio M, Scielzo C, Bertilaccio MT, Frenquelli M, Ghia P, Caligaris-Cappio F. Expression and function of toll like receptors in chronic lymphocytic leukaemia cells. Br J Haematol. 2009;144(4):507-516.
doi pubmed - Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101(11):4500-4504.
doi pubmed - Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102(3):956-963.
doi pubmed - Arvaniti E, Ntoufa S, Papakonstantinou N, Touloumenidou T, Laoutaris N, Anagnostopoulos A, Lamnissou K, et al. Toll-like receptor signaling pathway in chronic lymphocytic leukemia: distinct gene expression profiles of potential pathogenic significance in specific subsets of patients. Haematologica. 2011;96(11):1644-1652.
doi pubmed - Komatsuda A, Wakui H, Iwamoto K, Harada M, Okumoto Y, Sawada K. Gene expression profiling of peripheral blood mononuclear cells from patients with minimal change nephrotic syndrome by cDNA microarrays. Am J Nephrol. 2008;28(4):539-547.
doi pubmed - Midgley A, Thorbinson C, Beresford MW. Expression of Toll-like receptors and their detection of nuclear self-antigen leading to immune activation in JSLE. Rheumatology (Oxford). 2012;51(5):824-832.
doi pubmed - O'Mahony DS, Pham U, Iyer R, Hawn TR, Liles WC. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci. 2008;5(1):1-8.
doi pubmed - Ignatz-Hoover JJ, Wang H, Moreton SA, Chakrabarti A, Agarwal MK, Sun K, Gupta K, et al. The role of TLR8 signaling in acute myeloid leukemia differentiation. Leukemia. 2015;29(4):918-926.
doi pubmed - van den Ancker W, van Luijn MM, Ruben JM, Westers TM, Bontkes HJ, Ossenkoppele GJ, de Gruijl TD, et al. Targeting Toll-like receptor 7/8 enhances uptake of apoptotic leukemic cells by monocyte-derived dendritic cells but interferes with subsequent cytokine-induced maturation. Cancer Immunol Immunother. 2011;60(1):37-47.
doi pubmed - Rybka J, Butrym A, Wrobel T, Jazwiec B, Stefanko E, Dobrzynska O, Poreba R, et al. The expression of Toll-like receptors and development of severe sepsis in patients with acute myeloid leukemias after induction chemotherapy. Med Oncol. 2014;31(12):319.
doi pubmed - Rybka J, Butrym A, Wrobel T, Jazwiec B, Stefanko E, Dobrzynska O, Poreba R, et al. The expression of Toll-like receptors in patients with acute myeloid leukemia treated with induction chemotherapy. Leuk Res. 2015;39(3):318-322.
doi pubmed - Liang X TJ, Miller JS, LeBien TW, Blazar BR, Chen W. Toll-like receptor 7 agonist induces apoptosis of human B-lineage acute lymphocytic leukemia cells in vitro and in vivo ASCO annual meeting 2008.
- Reid GS, She K, Terrett L, Food MR, Trudeau JD, Schultz KR. CpG stimulation of precursor B-lineage acute lymphoblastic leukemia induces a distinct change in costimulatory molecule expression and shifts allogeneic T cells toward a Th1 response. Blood. 2005;105(9):3641-3647.
doi pubmed - Xu N, Yao HP, Lv GC, Chen Z. Downregulation of TLR7/9 leads to deficient production of IFN-alpha from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm Res. 2012;61(9):997-1004.
doi pubmed - Spaner DE, Shi Y, White D, Mena J, Hammond C, Tomic J, He L, et al. Immunomodulatory effects of Toll-like receptor-7 activation on chronic lymphocytic leukemia cells. Leukemia. 2006;20(2):286-295.
doi pubmed - Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, Chen W. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115(24):5041-5052.
doi pubmed - Muzio M, Fonte E, Caligaris-Cappio F. Toll-like Receptors in Chronic Lymphocytic Leukemia. Mediterr J Hematol Infect Dis. 2012;4(1):e2012055.
doi pubmed - Barcellini W, Imperiali FG, Zaninoni A, Reda G, Consonni D, Fattizzo B, Lonati S, et al. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: relationship with infections, autoimmunity and disease progression. Leuk Lymphoma. 2014;55(8):1768-1773.
doi pubmed - Klonowska-Szymczyk A, Wolska A, Robak T, Cebula-Obrzut B, Smolewski P, Robak E. Expression of toll-like receptors 3, 7, and 9 in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Mediators Inflamm. 2014;2014:381418.
doi pubmed - Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840-1848.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


