| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 5, Number 4, December 2016, pages 129-137
Ebstein-Barr Virus-Negative Clonal Plasma Cell Proliferations Associated With Systemic Involvement of Primary Cutaneous T-Cell Lymphoma
Smita C. Patela, b, Brett Mahona
aDepartment of Pathology, Rush University Medical Center, Chicago, IL, USA
bCorresponding Author: Smita C. Patel, Department of Pathology, Rush University Medical Center, 1750 W. Harrison St, Rm 570 Jelke, Chicago, IL 60612, USA
Manuscript accepted for publication November 04, 2016
Short title: EBV-Negative Clonal Plasma Cell Proliferation
doi: https://doi.org/10.14740/jh300w
| Abstract | ▴Top |
We describe a 66-year-old man with a long-standing history of squamous cell carcinoma of the head and neck region who developed nodular-sclerosing subtype of classical Hodgkin lymphoma and primary cutaneous CD8-positive, cytotoxic variant of mycosis fungoides over a 1-year period of time. Within a few months of his diagnosis of primary cutaneous T-cell lymphoma, he developed systemic involvement of T-cell lymphoma in an axillary lymph node, bone marrow and lung. Interestingly, his lung infiltrates had an Ebstein-Barr virus (EBV)-negative, mature clonal plasma cell proliferations intermingled with neoplastic T cells. It showed cytoplasmic κ light chain restriction by in situ hybridization and also revealed clonal immunoglobulin light chain rearrangements in both κ and λ. No morphological or immunohistochemical evidence of his prior Hodgkin lymphoma was identified in the bone marrow or lung. In the short available follow-up (2 months to date), the patient is doing well and being evaluated for possible allogeneic stem cell transplant. The association of EBV-positive B-cell expansions in T-cell lymphomas, especially angioimmunoblastic T-cell lymphoma, is well recognized. However, EBV-negative, clonal B-cell or plasma cell proliferations in T-cell lymphoma may represent a specific phenomenon and its significance still needs further clarification.
Keywords: Peripheral T-cell lymphomas; Plasma cell proliferations; Clonal; Ebstein-Barr virus
| Introduction | ▴Top |
Peripheral T-cell lymphomas (PTCLs) are a functionally and morphologically heterogeneous group of aggressive lymphoid neoplasms characterized by clonal proliferations of T cells, associated with variable numbers of reactive cells including non-neoplastic T cells, reactive B lymphocytes, histiocytes and other inflammatory cells [1, 2]. This non-neoplastic population may sometimes be very extensive, obscuring the tumor cell population [3]. There has recently been greater recognition of B-cell expansions that can be a component of these tumors [4]. Hodgkin and Reed-Sternberg (HRS)-like cells of B-cell lineage have been described in PTCL, especially in cases showing features of angioimmunoblastic T-cell lymphoma (AITL), in which a population of large B cells, infected with Ebstein-Barr virus (EBV), is invariably present [1, 5]. EBV has been found in 43% of PTCLs in the Caucasian population [6]. EBV presence in AITL was first reported by Bornkamm et al in 1976 [7]. EBV plays an important role in the early development of B-cell proliferations in PTCL and further progression of these clones into aggressive B-cell neoplasms. These B-cell populations show a spectrum of morphology ranging from small, isolated clusters of large activated B cells to focally confluent transformed B cells [8-10]. It was postulated that the expansion of EBV-positive B cells was related to defective immune surveillance secondary to underlying T-cell malignancy [8, 10-15]. Molecular studies have demonstrated oligoclonal and clonal B-cell rearrangements in up to 40% of AITLs [16-19] that are not always clearly related to morphologically transformed B cells. The role of EBV in B-cell proliferations is well understood in AITL, but to a lesser degree in other PTCLs.
Subsequently, EBV-negative HRS-like cells have been recognized in cases of PTCL [4, 20]. Nicolae et al described that the phenomenon of EBV-negative HRS-like cells in PTCL cannot be attributed solely to defective surveillance for EBV, and suggested other mechanisms for abnormal B-cell proliferations [4]. Brauninger et al observed an EBV-negative B-cell clone expanding in one case of AITL [12] and Lome-Maldonado et al identified one case of AITL with high number of clonal large B cells which were EBV-negative [9]. Balague et al studied 15 patients with PTCL and associated EBV-negative clonal or monotypic B-cell proliferations which ranged from plasma cell proliferations to overt lymphomas with plasmacytic/plasmablastic features [21]. However, the phenomenon of having EBV-negative B-cell proliferations in PTCL is extremely uncommon and therefore, the clinicopathological characteristics of these patients and the malignant potential of these B-cell proliferations are not well understood.
Here, we describe a 66-year-old man who developed systemic involvement of CD8-positive, cytotoxic T-cell variant of cutaneous mycosis fungoides and an associated clonal plasma cell proliferation intermingled with neoplastic T cells in the lung infiltrates.
| Case Report | ▴Top |
A 66-year-old man had a past history of squamous cell carcinoma of the head and neck region which was treated with surgical resection and postoperative radiation at an outside institution. Three years later, he presented to our institution with complaints of ulceration in the left post-auricular area over the mastoid, intermittent twitching of his lower face, worsening of his marginal mandibular nerve weakness, and minimal weight loss. Physical examination revealed a 3.0 × 0.5 cm ulcerative mass with induration in the post-auricular area. There was no evidence of adenopathy. A subsequent CT scan of the neck revealed an ill-defined, heterogeneously enhancing and infiltrative soft tissue in the left infra-mastoid region with extension into the carotid, para-pharyngeal space, para-spinal musculature and parotid gland with thickening of the overlying skin and subcutaneous soft tissues. There was no evidence of adenopathy or any distant metastasis on imaging. The patient underwent a complex resection including radical parotidectomy and partial auriculectomy with nerve grafting and regional flap reconstruction. During the operation, an incidental small nodule within the soft tissue on the neck musculature was seen and excised. The main surgical specimen showed an invasive moderately differentiated squamous cell carcinoma with free resection margins. However, the incidentally found nodule within the soft tissue of neck showed nodular effacement of normal lymph node architecture with areas of sclerosis around the nodules and a thickened nodal capsule. Scattered HRS cells were identified, mixed with reactive small lymphocytes and to a lesser degree, neutrophils and histiocytes. Occasional lacunar cells were also present. By immunohistochemistry (IHC), these HRS cells were positive for CD15, CD30, CD79a, EBV, weakly positive for Pax-5 and were negative for CD45, CD3 and CD20. The morphological findings and immunophenotype were consistent with a nodular sclerosing subtype of classical Hodgkin lymphoma. The staging bone marrow biopsy was deferred at that time. The patient received high doses of radiation therapy for his squamous cell carcinoma, which was thought to be reasonably curative for Hodgkin lymphoma as well. On completion of radiation, patient underwent staging PET-CT scans which revealed no residual disease and patient was thought to be in remission.
Within 5 months of his initial presentation at our institution, the patient developed multiple sites of skin involvement by in situ squamous cell carcinoma. During his regular follow-ups with dermatology clinic, he had developed diffuse and scattered erythematous, excoriated, xerotic, and scaly patches over bilateral arms, face and abdomen. A punch biopsy of one of these lesions showed dense, atypical lymphoid infiltrate arranged in a band-like configuration filling the superficial dermis with mild to moderate epidermotropism as well as infiltrating around and into the adnexal structures (Fig. 1A, B). The lymphoid cells were small to medium-sized and had mature clumped chromatin and cerebriform nuclear contours (Fig. 1C). By IHC, these atypical lymphoid cells were positive for CD3 (Fig. 1D), CD5 and weak CD8 (Fig. 1F) with partial loss of CD7. The neoplastic cells were negative for CD4 (Fig. 1E), CD20 and CD30. The cytotoxic associated proteins including granzyme B and TIA-1 were positive in neoplastic cells (Fig. 1G and H, respectively). The neoplastic cells showed diffuse labeling for TCR-beta. The absence of necrosis, ulceration, spongiotic microvesiculation and marked epidermotropism favored a CD8-positive, cytotoxic immunophenotypic variant of mycosis fungoides over primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma according to 2005 WHO/EORTC classification for cutaneous lymphomas.
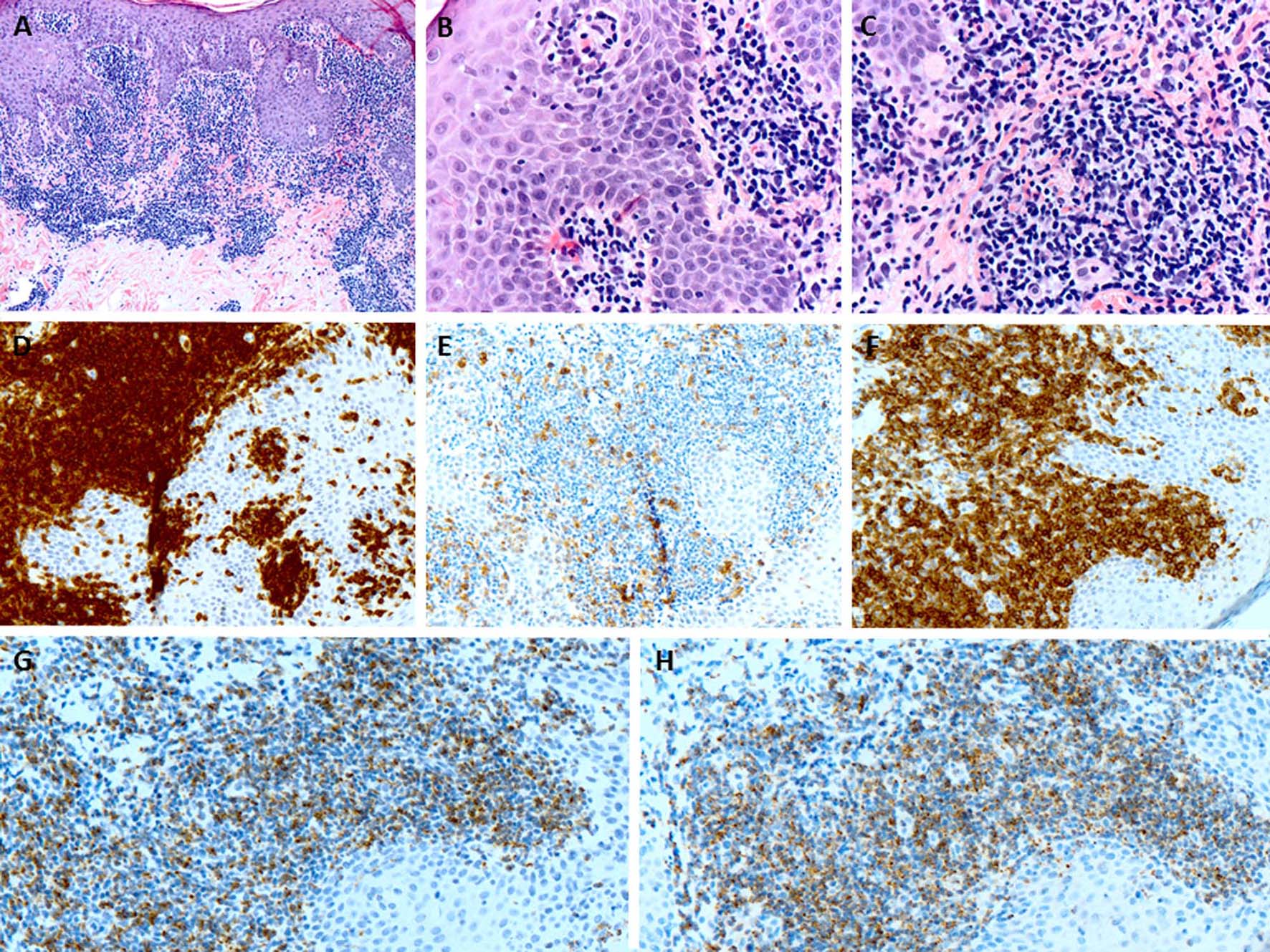 Click for large image | Figure 1. (A) The punch biopsy of the skin lesion shows a dense atypical lymphoid infiltrate predominantly in the dermis (H&E, × 100). (B) The lymphoid infiltrate shows minimal epidermotropism (H&E, × 400). (C) The lymphoid cells are small to medium-sized and have mature chromatin and irregular nuclear contours (H&E, × 400). (D) The lymphoid cells are CD3-positive T cells (× 200). (E) CD4 shows minimal scattered staining (× 200). (F) The atypical cells largely positive for CD8 (× 200). (G, H) The cytotoxic proteins including granzyme B and TIA-1 are positive in atypical lymphoid cells respectively (× 200). |
A few days later, the patient presented with palpable axillary lymph nodes. Subsequent PET-CT scan revealed hypermetabolic right axillary lymph nodes as well as hypermetabolic nodules in the right lower lung. The excisional biopsy of the axillary lymph node showed complete effacement of the lymph node architecture by small to medium-sized mature lymphocytes with a diffuse pattern (Fig. 2A). Focally, these lymphoid cells had irregular nuclear contours. Loose clusters of larger “lacunar” type Hodgkin cells were also identified (Fig. 2B). By IHC, the Hodgkin cells were positive for Pax5 (Fig. 2D), CD30 (Fig. 2E), CD15 and EBV (Fig. 2F). CD20 was positive only in scattered small B lymphocytes but the Hodgkin cells were negative. All of the remaining smaller lymphocytes were diffusely positive for CD3 (Fig. 2C). Flow cytometry identified a large population (about 85% of the total cells) of small to medium-sized T cells expressing CD3, CD2, CD5, and TCR alpha/beta, dim CD8 with variable expression of CD57 and partial loss of CD7. This population was lacking CD4, CD30 and CD56. The overall morphological and immunophenotypic findings were consistent with a composite lymphoma, composed of classical Hodgkin lymphoma in a background of a mature T-cell lymphoma. Polymerase chain reaction (PCR) for TCR-γ gene rearrangement was positive and confirmed the clonality of T cells. Because of hypermetabolic nodules in the right lung, a wedge biopsy of the right lower lobe of the lung was performed. The microscopic sections showed a diffuse infiltrate of neoplastic, small to medium-sized T cells predominantly around vessels, bronchi and alveolar walls (Fig. 3A). Interestingly, in addition to the T cells, sheets and clusters of plasma cells were also present (Fig. 3B). Morphologically, the plasma cells were mature with occasional atypical features such as larger and irregular nuclei and prominent nucleoli. The plasma cells were present admixed with the neoplastic T cells. By IHC, the lymphoid infiltrate was composed of CD3-positive (Fig. 3C) and CD5-positive T cells which were focally and weakly positive for CD8 (Fig. 3D) and negative for CD4. Flow cytometric analysis showed a mature T-cell lymphoma with a similar immunophenotype as identified in prior skin and axillary lymph node specimens (it showed approximately 71% of total cells being TCR alpha/beta T cells with expression of CD3, CD2, CD5, partial loss of CD7, variable CD57, and dim CD8 and lacking CD4, CD30 and CD56). CD138 recapitulated the morphological evidence of clusters and sheets of mature plasma cells (Fig. 4A, B) which were negative for CD56, cyclin-D1, CD20 and EBV (Fig. 4E). In situ hybridization (ISH) revealed monotypic κ light chain restriction of the plasma cells (Fig. 4C) with a paucity of λ light chains (Fig. 4D). No morphological or immunohistochemical evidence of Hodgkin lymphoma was identified in the lung tissue. PCR for TCR-γ gene rearrangement was positive on the lung tissue sample (Fig. 5). Since the plasma cell component showed monotypic κ light chain restriction, PCR for light chain immunoglobulin gene rearrangement was also performed and showed clear monoclonal bands in both κ and λ light chains (Fig. 6). The concurrent bone marrow biopsy showed multifocal mature T-cell lymphoma (Fig. 7A, B); however, no evidence of Hodgkin lymphoma as well as no increased or atypical plasma cell component (Fig. 7C-E) was identified. As of date, the patient is doing well and being evaluated for allogeneic stem cell transplant as a possible therapeutic option.
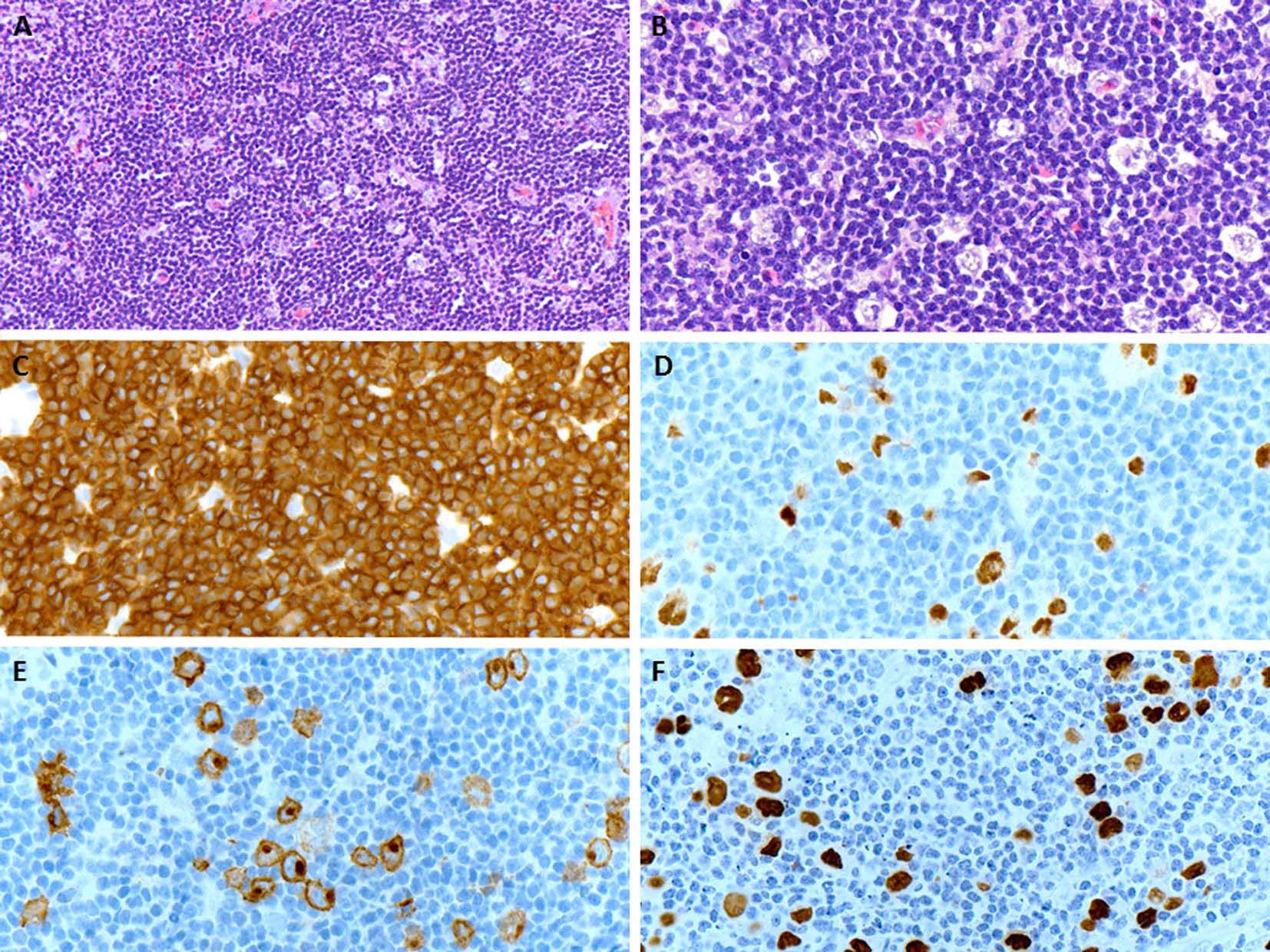 Click for large image | Figure 2. (A) An excisional biopsy of the axillary lymph node shows a nodal effacement by diffuse infiltrate of small and mature lymphocytes (H&E, × 200). (B) Loose clusters of larger “lacunar” type Hodgkin cells are also appreciated on higher magnification (H&E, × 400). (C) The neoplastic population of small and mature lymphocytes are diffusely positive for CD3 (× 400). (D, E) The large Hodgkin cells are positive for Pax5 and CD30 (membranous and Golgi staining), respectively (× 400). (F) The in situ hybridization for EBV is positive in Hodgkin cells (× 400). |
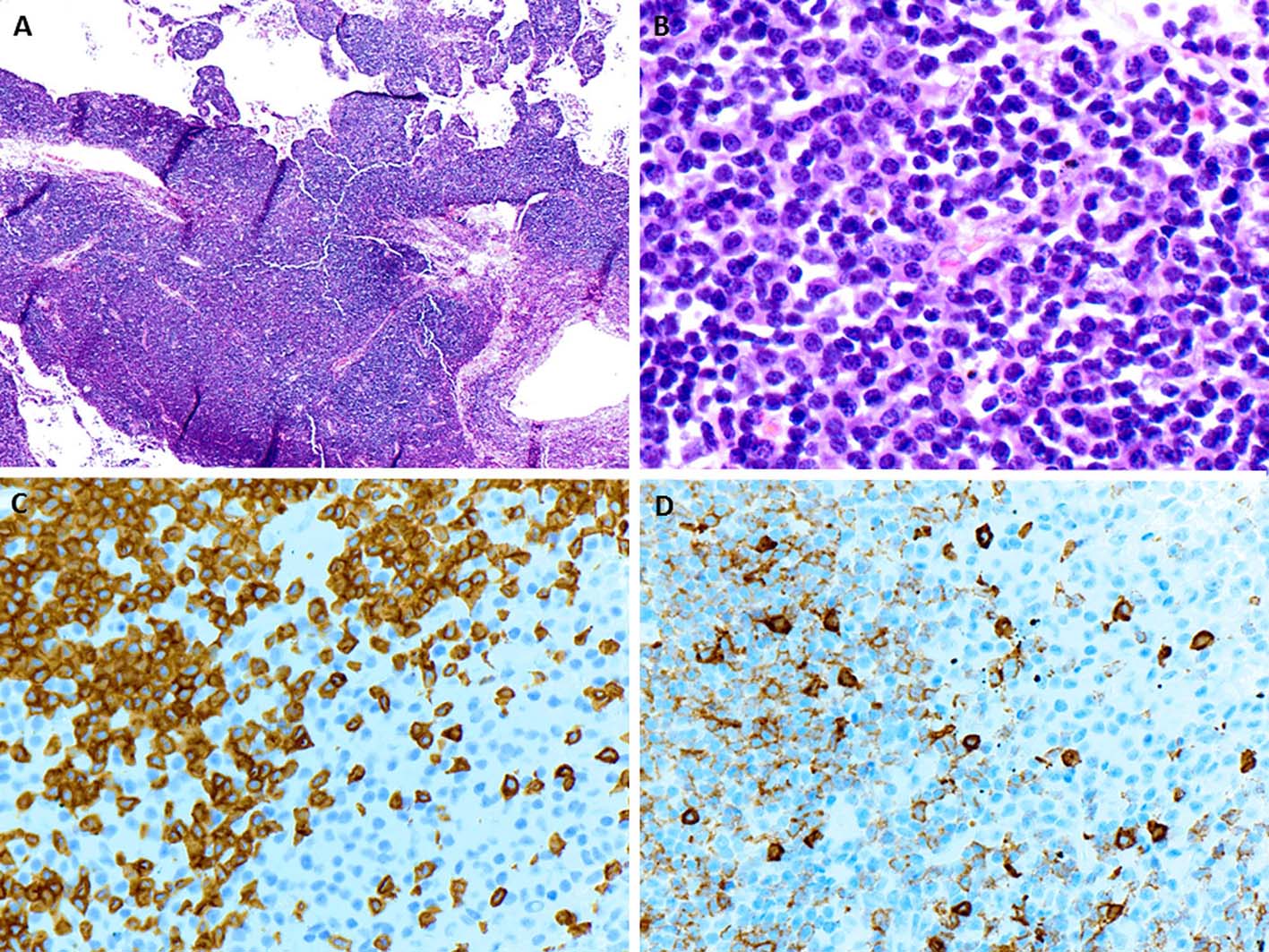 Click for large image | Figure 3. (A) The wedge resection of right lower lobe of lung shows a diffuse infiltrate predominantly around vessels, bronchi and alveolar walls (H&E, × 40). (B) On higher magnification, loose clusters of mature plasma cells are intimately associated with neoplastic population of mature lymphocytes (H&E, × 600). (C, D) The neoplastic lymphocytes are positive for CD3 and weakly for CD8 respectively while the clusters of plasma cells are negative for both (× 400). |
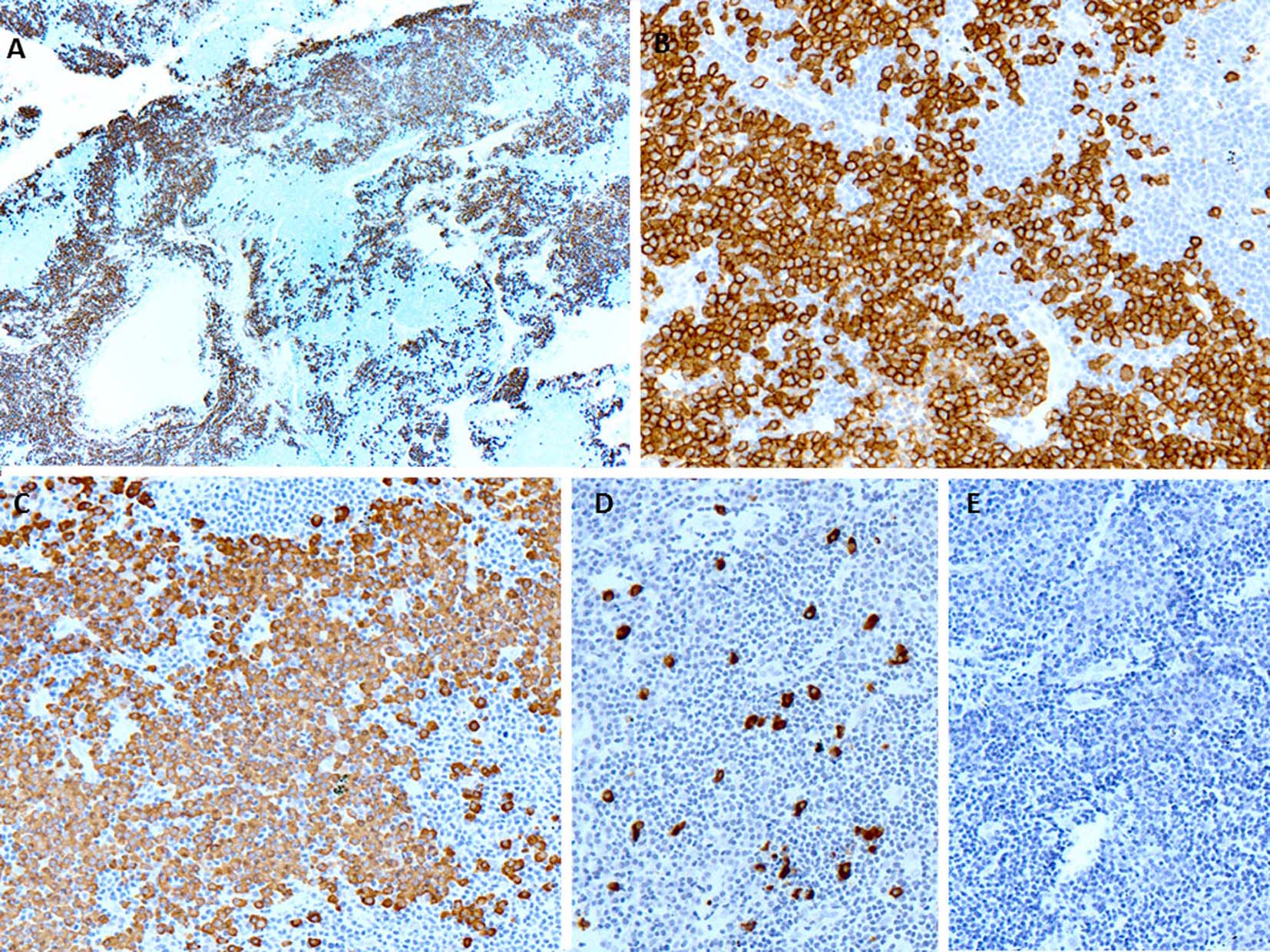 Click for large image | Figure 4. (A, B) CD138 immunostaining shows large clusters and sheets of plasma cells closely associated with negative neoplastic T lymphocytic population (× 40 and × 200, respectively). (C) The plasma cells show monotypic κ light chains by in situ hybridization (× 200). (D) The in situ hybridization for λ light chains shows marked paucity (× 200). (E) The in situ hybridization for EBV is negative (× 200). |
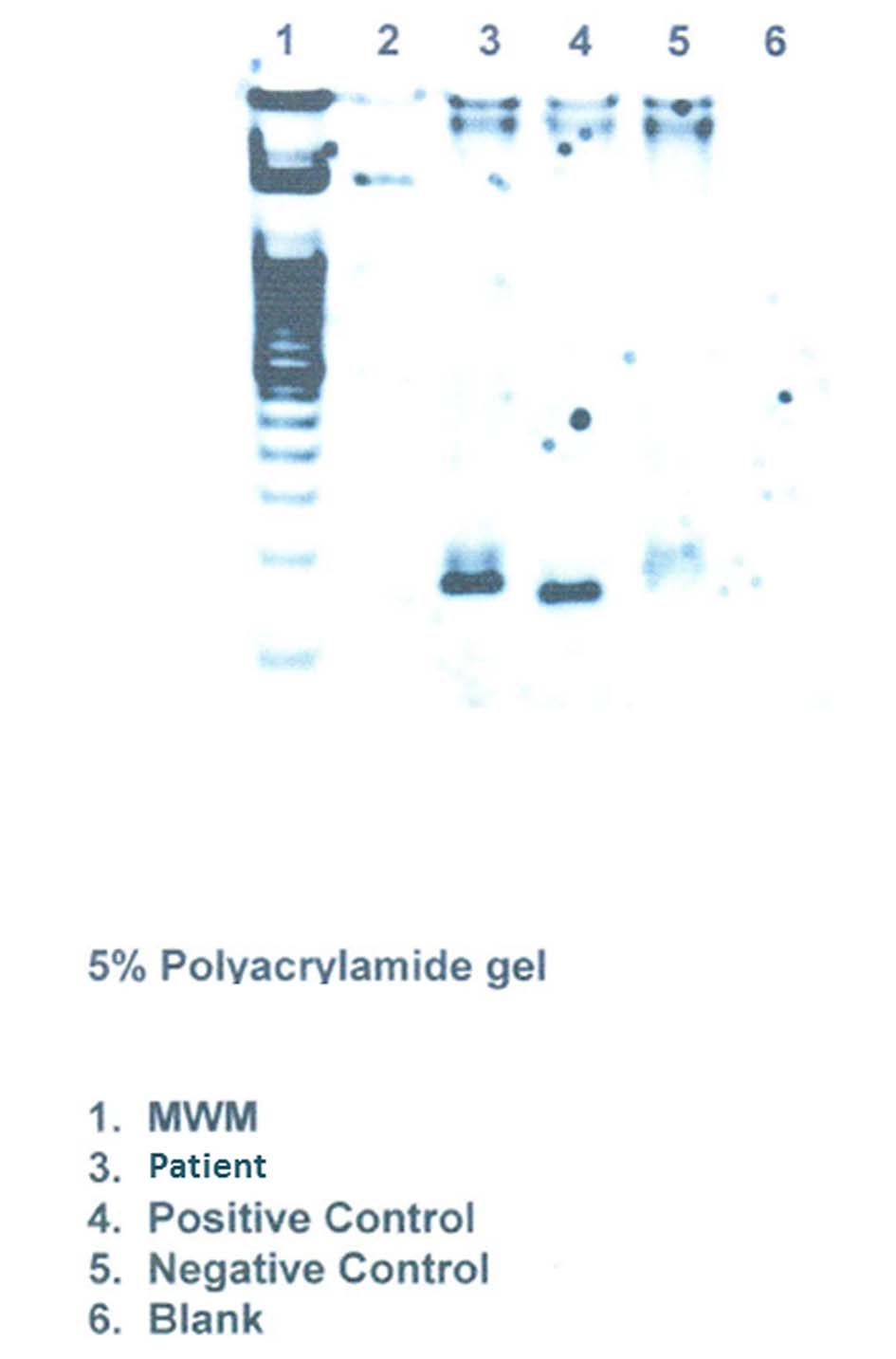 Click for large image | Figure 5. Clonality studies on the lung tissue shows TCR-γ gene rearrangement. |
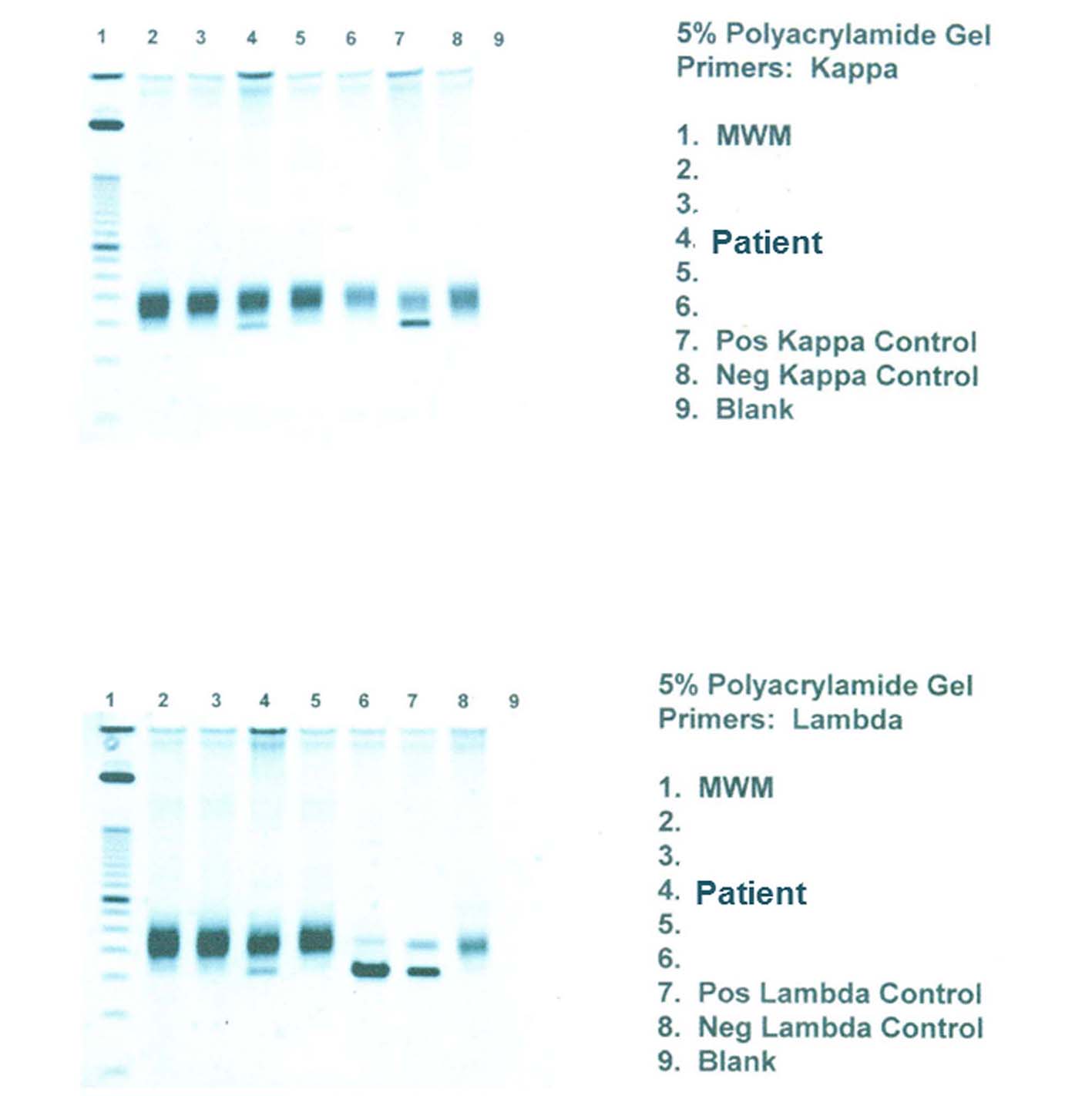 Click for large image | Figure 6. PCR for light chain immunoglobulin gene rearrangement shows clear monoclonal bands in both κ and λ light chains. |
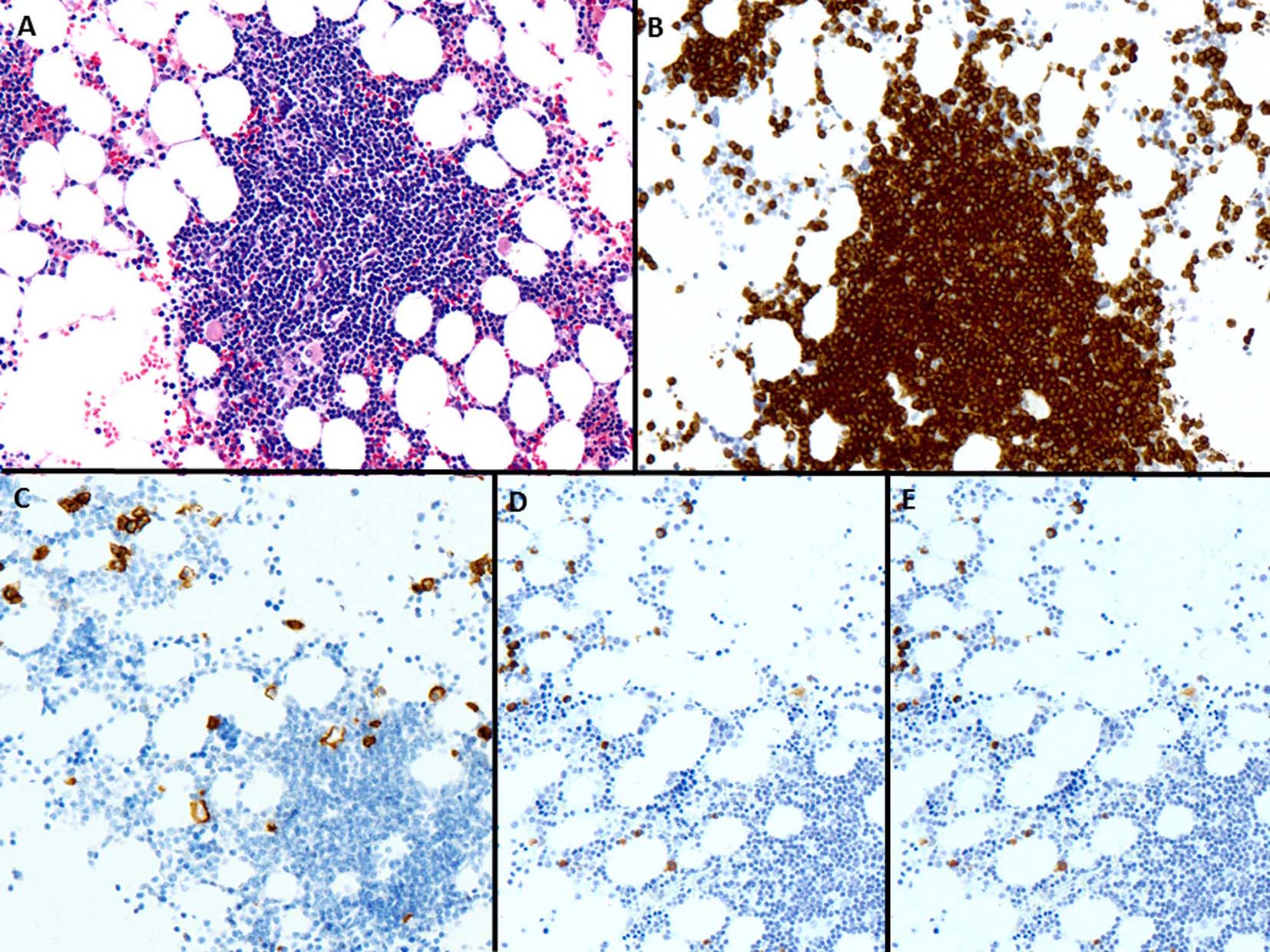 Click for large image | Figure 7. (A) The bone marrow biopsy shows infiltration by small and mature lymphocytes (H&E, × 200). (B) CD3 is positive in lymphoid cells (× 200). (C) CD138 shows few scattered plasma cells (× 200). (D, E) The plasma cells are polyclonal by κ and λ in situ hybridization, respectively (× 200). |
| Discussion | ▴Top |
PTCLs have been previously described to contain B-cell proliferations. However, most of these B-cell proliferations have been associated with EBV and have large cell or HRS-like cell morphology [8-10, 15, 22, 23]. It was hypothesized that EBV-positive cells were expanded due to defects in immune surveillance [10-15]. In addition to this well-established relationship between EBV-associated B-cell proliferations and PTCL, rare case reports and small series exist in the literature describing EBV-negative B-cell proliferations in various PTCLs [4, 24]. Nicolae et al assessed the nature of the PTCL associated with HRS-like cells to determine whether EBV-negative HRS-like cells may be seen [4]. They identified 57 cases of PTCL and among them, 52 cases were associated with EBV. They included five cases in which the B-cell expansions were EBV-negative, three classified as AITL and two as PTCL-NOS, follicular variant. Thus, all EBV-negative cases had a TFH-immunophenotype. Two pathways were hypothesized. First, HRS-like cells could be driven by the microenvironment. All of their EBV-negative cases had CD4-positive, PD-1-positive neoplastic T cells intimately rosetting the HRS-like cells and thus, TFH cells may play a critical role in the generation of T-cell dependent B-cell responses, and promote the expansion of B cells in the immune response. A second hypothesis suggested that the virus might be seen very early in the course of the disease and might persist or disappear during disease progression. The clinical significance of EBV-negative B-cell proliferations and its malignant potential in the context of PTCL is still uncertain.
Additionally, the large majority of PTCL cases with co-existence of B-cell expansions in the literature happen to be AITL with rare cases of PTCL, NOS and adult T-cell leukemia lymphoma [4, 8, 9, 19, 25-27]. Cutaneous T-cell lymphomas have rarely been described to be associated with B-cell expansions [21, 28] and if so, mostly as case reports and small case series. Most of these reports describe associated and established B-cell malignancies rather than clonal non-neoplastic proliferations. Barzilai et al [28] in their series summarized 17 prior reports and reported 11 additional patients with a previous diagnosis of mycosis fungoides who later developed B-cell malignancies. The subsequent B-cell malignancies in these 28 patients included 16 non-Hodgkin lymphomas, six chronic lymphocytic leukemias, five myelomas, and one hairy cell leukemia. Interestingly, none of the patients developed Hodgkin lymphoma. However, the B-cell malignancies in these patients either developed later or preceded the diagnosis of mycosis fungoides. The time elapsed between the onset of the two malignancies ranged from 4 to 22 years (average: 12 years). Additionally, the association and role of EBV in the development of B-cell malignancies is not mentioned.
Almost all B-cell disorders described in PTCL have large cell, HRS-like morphology or large-cell lymphomas with a mature immunophenotype. The plasma cell morphology in the context of PTCL is highly unusual. Only one case report of EBV-positive nodal plasmacytoma after 8 years of the initial diagnosis of AITL has been reported in the literature [10]. Various lymphoid neoplasms with plasmacytic differentiation have been described in association with immunodeficiency such as HIV infection or post-organ transplant. However, these cases are usually EBV-associated and show aggressive, plasmablastic features contrary to the mature cell morphology of plasma cells [21]. Mature plasma cell-rich expansions have also been observed as an uncommon subtype of post-transplant lymphoproliferative disorders that may respond to the reduction of immunosuppression [29]; however, these cases are usually EBV-positive and may progress to aggressive plasmablastic lymphomas [30]. Our case is highly unusual since the patient had a history of Hodgkin lymphoma and primary cutaneous T-cell lymphoma, then subsequently developed an EBV-negative, clonal mature plasma cell proliferations admixed with neoplastic T cells in an extra-nodal site. No morphological or immunohistochemical evidence of HRS-like cells or large cells was present. A search of the English language literature revealed only one isolated case report of AITL and one small series of various PTCL describing intermingled EBV-negative plasma cell proliferations similar to our patient [21, 24]. Balague et al [21] identified 15 patients including three cutaneous T-cell lymphomas with EBV-negative clonal or monotypic B-cell proliferations arising in the context of PTCL. Their series described striking plasma cell differentiation of the B-cell expansions in contrast to the large cell or HRS-like morphology of EBV-related B-cell proliferations and lymphoid neoplasms observed in PTCL. The B-cell component was intermingled with the PTCL in all of their patients, as identified in our case. It was classified as clonal/monotypic plasma cell proliferations in eight lesions, clonal/monotypic large B-cell proliferations in four cases, and a B-cell lymphoma with plasmacytic/plasmablastic differentiation in three patients. Sequential samples in seven of 15 patients showed persistence of the PTCL and the B-cell component in four, the PTCL without the B-cell lymphoma in two, and progression of the B-cell neoplasm in one. The eight patients with clonal/monotypic plasma cell proliferations included three PTCL-NOS, two AITL, two cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphomas and one cutaneous PTCL-NOS. The majority of these patients presented with skin lesions while one had lung infiltrates, as in our case. Interestingly, one of these patients had different types of light chain restriction at two different relapsed sites, λ in the subcutaneous tissue and κ in the lung infiltrates. At the last follow-up, two patients were alive without disease, three had evidence of local and disseminated disease and one died of extensive disease. Among the group of 15 patients, three cases had associated plasmacytic/plasmablastic lymphoma which followed an aggressive clinical course.
The evidence suggests differences in EBV-negative B-cell proliferations compared to EBV-driven B-cell disorders in those EBV-negative B-cell expansions are more common in females, have frequent extra-nodal presentations, and they were recognized simultaneously or only after 9 months of the initial diagnosis [21]. It has been suggested that EBV-negative B-cell proliferations in AITL and other PTCLs are more dependent on the T-cell component and subsequently more unstable and sensitive to treatment [21]. The B-cell expansions in PTCL with TFH-cell derivation and AITL can be explained by the role of intimately rosetted, PD-1-positive neoplastic T cells, but it is entirely unclear about the mechanism of preferential plasma cell differentiation of B cells, especially in cases of cutaneous T-cell lymphomas and other PTCL.
In our case, having an extensive plasma cell proliferation in the lung tissue led us to consider a plasma cell neoplasm or a marginal zone lymphoma with extensive plasmacytic differentiation unrelated to the patient’s cutaneous T-cell lymphoma. The features arguing against this hypothesis include very minimal and polyclonal plasma cells in the bone marrow (typical of a plasma cell neoplasm) and the lack of a clonal and monocytoid B-cell component (typical of a marginal zone lymphoma). κ light restriction and clonal rearrangement of immunoglobulin light chains also point towards the diagnosis of an extraosseous plasmacytoma; however, the lack of a solid mass consisting of sheets of plasma cells, the close association of the plasma cell proliferations to the neoplastic T-cells, and the overall characteristics are consistent with these proliferations being associated with the patient’s T-cell lymphoma.
In conclusion, we report a case of primary CD8-positive cytotoxic variant of cutaneous T-cell lymphoma with clonal EBV-negative, mature plasma cell proliferations in association with the systemic involvement by T-cell lymphoma. There is a well-established relationship between EBV-positive B-cell proliferations and PTCL; however, EBV-negative plasma cell proliferations associated with PTCL have not been frequently reported. The distinctive pathologic features of these lesions and the particularity of some clinical aspects suggest that they may represent a specific phenomenon developed in a subset of PTCL. It is important to avoid the misdiagnosis of a plasma cell neoplasm which may result in dramatic change in the further management of the patient. Although rare cases were reported to progress to aggressive B-cell neoplasms [21], more case reports and larger studies are required to clarify the clinical significance and malignant potential of these proliferations, which at present is still unknown.
Conflicts of Interest
None.
Funding
None.
| References | ▴Top |
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon: International Agency of Research on Cancer (IARC); 2008.
- Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, Nathwani BN, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 2002;13(1):140-149.
doi pubmed - Geissinger E, Bonzheim I, Krenacs L, Roth S, Strobel P, Ott G, Reimer P, et al. Identification of the tumor cells in peripheral T-cell lymphomas by combined polymerase chain reaction-based T-cell receptor beta spectrotyping and immunohistological detection with T-cell receptor beta chain variable region segment-specific antibodies. J Mol Diagn. 2005;7(4):455-464.
doi - Nicolae A, Pittaluga S, Venkataraman G, Vijnovich-Baron A, Xi L, Raffeld M, Jaffe ES. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. Am J Surg Pathol. 2013;37(6):816-826.
doi pubmed - Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, Meijer CJ, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008;111(9):4463-4470.
doi pubmed - Ho JW, Ho FC, Chan AC, Liang RH, Srivastava G. Frequent detection of Epstein-Barr virus-infected B cells in peripheral T-cell lymphomas. J Pathol. 1998;185(1):79-85.
doi - Bornkamm GW, Stein H, Lennert K, Ruggeberg F, Bartels H, zur Hausen H. Attempts to demonstrate virus-specific sequences in human tumors. IV. EB viral DNA in European Burkitt lymphoma and immunoblastic lymphadenopathy with excessive plasmacytosis. Int J Cancer. 1976;17(2):177-181.
doi pubmed - Higgins JP, van de Rijn M, Jones CD, Zehnder JL, Warnke RA. Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J Clin Pathol. 2000;114(2):236-247.
doi pubmed - Lome-Maldonado C, Canioni D, Hermine O, Delabesse E, Damotte D, Raffoux E, Gaulard P, et al. Angio-immunoblastic T cell lymphoma (AILD-TL) rich in large B cells and associated with Epstein-Barr virus infection. A different subtype of AILD-TL? Leukemia. 2002;16(10):2134-2141.
doi pubmed - Zettl A, Lee SS, Rudiger T, Starostik P, Marino M, Kirchner T, Ott M, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. 2002;117(3):368-379.
doi pubmed - Abruzzo LV, Schmidt K, Weiss LM, Jaffe ES, Medeiros LJ, Sander CA, Raffeld M. B-cell lymphoma after angioimmunoblastic lymphadenopathy: a case with oligoclonal gene rearrangements associated with Epstein-Barr virus. Blood. 1993;82(1):241-246.
pubmed - Brauninger A, Spieker T, Willenbrock K, Gaulard P, Wacker HH, Rajewsky K, Hansmann ML, et al. Survival and clonal expansion of mutating "forbidden" (immunoglobulin receptor-deficient) epstein-barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med. 2001;194(7):927-940.
doi pubmed - Attygalle AD, Kyriakou C, Dupuis J, Grogg KL, Diss TC, Wotherspoon AC, Chuang SS, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol. 2007;31(7):1077-1088.
doi pubmed - Willenbrock K, Brauninger A, Hansmann ML. Frequent occurrence of B-cell lymphomas in angioimmunoblastic T-cell lymphoma and proliferation of Epstein-Barr virus-infected cells in early cases. Br J Haematol. 2007;138(6):733-739.
doi pubmed - Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992;79(7):1789-1795.
pubmed - Feller AC, Griesser H, Schilling CV, Wacker HH, Dallenbach F, Bartels H, Kuse R, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133(3):549-556.
pubmed - Frizzera G, Kaneko Y, Sakurai M. Angioimmunoblastic lymphadenopathy and related disorders: a retrospective look in search of definitions. Leukemia. 1989;3(1):1-5.
pubmed - Lipford EH, Smith HR, Pittaluga S, Jaffe ES, Steinberg AD, Cossman J. Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma. J Clin Invest. 1987;79(2):637-642.
doi pubmed - Smith JL, Hodges E, Quin CT, McCarthy KP, Wright DH. Frequent T and B cell oligoclones in histologically and immunophenotypically characterized angioimmunoblastic lymphadenopathy. Am J Pathol. 2000;156(2):661-669.
doi - Warnke RA, Jones D, Hsi ED. Morphologic and immunophenotypic variants of nodal T-cell lymphomas and T-cell lymphoma mimics. Am J Clin Pathol. 2007;127(4):511-527.
doi pubmed - Balague O, Martinez A, Colomo L, Rosello E, Garcia A, Martinez-Bernal M, Palacin A, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. 2007;31(9):1310-1322.
doi pubmed - Anagnostopoulos I, Hummel M, Finn T, Tiemann M, Korbjuhn P, Dimmler C, Gatter K, et al. Heterogeneous Epstein-Barr virus infection patterns in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type. Blood. 1992;80(7):1804-1812.
pubmed - Quintanilla-Martinez L, Fend F, Moguel LR, Spilove L, Beaty MW, Kingma DW, Raffeld M, et al. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol. 1999;23(10):1233-1240.
doi pubmed - Huppmann A, Roullet M, Jaffe E, et al. Angioimmunoblastic T-cell lymphoma partially obscured by an EBV-negative clonal plasma cell proliferation. Journal of Clinical Oncology. 2013;31(2).
doi pubmed - Brenton T. Tan, Roger A. Warnke, Daniel A. Arber. The Frequency of B- and T-Cell GeneRearrangements and Epstein-Barr Virus in T-Cell Lymphomas. A Comparison Between Angioimmunoblastic T-Cell Lymphoma and Peripheral T-Cell Lymphoma, Unspecified With and Without Associated B-Cell Proliferations. Journal of Molecular Diagnostics. 2006;8(4).
- Moroch J, Copie-Bergman C, de Leval L, Plonquet A, Martin-Garcia N, Delfau-Larue MH, Molinier-Frenkel V, et al. Follicular peripheral T-cell lymphoma expands the spectrum of classical Hodgkin lymphoma mimics. Am J Surg Pathol. 2012;36(11):1636-1646.
doi pubmed - Venkataraman G, Berkowitz J, Morris JC, Janik JE, Raffeld MA, Pittaluga S. Adult T-cell leukemia/lymphoma with Epstein-Barr virus-positive Hodgkin-like cells. Hum Pathol. 2011;42(7):1042-1046.
doi pubmed - Barzilai A, Trau H, David M, Feinmesser M, Bergman R, Shpiro D, Schiby G, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155(2):379-386.
doi pubmed - Swerdlow SH. Classification of the posttransplant lymphoproliferative disorders: from the past to the present. Semin Diagn Pathol. 1997;14(1):2-7.
pubmed - Nelson BP, Nalesnik MA, Bahler DW, Locker J, Fung JJ, Swerdlow SH. Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000;24(3):375-385.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


