| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 6, Number 4, October 2017, pages 90-95
Infiltrative Rash Secondary to Leukemic-Phase Diffuse Large B-Cell Lymphoma With t(14;18), CDKN2A and MLL Deletion
Iris Y. Shenga, d, Diana O. Treabab, Kenneth D. Bishopc
aDepartment of Internal Medicine, Rhode Island Hospital and Warren Alpert Medical School, 593 Eddy Street, Providence, RI 02903, USA
bDepartment of Pathology and Laboratory Medicine, Rhode Island Hospital and Warren Alpert Medical School, 593 Eddy Street, Providence, RI 02903, USA
cDepartment of Hematology and Oncology, Sturdy Hospital, 211 Park St, Attleboro, MA 02703, USA
dCorresponding Author: Iris Y. Sheng, Department of Internal Medicine, Rhode Island Hospital and Warren Alpert Medical School, 593 Eddy Street, Providence, RI 02903, USA
Manuscript submitted July 23, 2017, accepted July 31, 2017
Short title: Leukemic-Phase DLBCL Presenting With a Rash
doi: https://doi.org/10.14740/jh327w
| Abstract | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous and highly aggressive subtype of non-Hodgkin’s lymphoma. It commonly presents as rapidly-growing, painless lymphadenopathy (LAD). DLBCL presenting in leukemic-phase is rare, with fewer than 40 cases published. Chemotherapy remains the standard approach, although selecting the correct regimen has become more perplexing in patients with CDKN2A mutations. Patients with MLL- and CDKN2A-positive DLBCL may benefit from therapy with a dose-adjusted regimen of rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-R-EPOCH) compared to traditional rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP). Herein, we report a case of leukemic-phase DLBCL presenting as a cutaneous eruption of the bilateral lower extremities, which has not been previously reported in the literature.
Keywords: Leukemic-phase DLBCL; MLL/CDKNA deletion
| Introduction | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is a rare and highly aggressive subtype of non-Hodgkin’s lymphoma (NHL) [1]. DLBCL is histologically characterized by large atypical lymphoid-appearing cells with pale blue cytoplasm; round, irregular, and vesicular nuclei with prominent nucleoli, and relatively abundant cytoplasm [2] commonly manifest as lymphadenopathy (LAD) of the neck, thorax, or abdomen. However, extra-nodal presentations have been described in the gastrointestinal tract, liver, spleen, and the skin [3]. There are numerous case reports of primary cutaneous DLBCL, leg type, defined as a primary cutaneous lymphoma with no organ or nodal involvement at the time of diagnosis [4]. Secondary lymphomatous cutaneous lesions involving the head, neck or trunk are estimated to be around 6% of DLBCL cases [5]. In general, skin manifestations indicate a poor prognosis, with an estimated 5-year overall survival (OS) of 43-63% [5]. There have been no reports of leukemic-phase DLBCL with skin manifestations to date, as this is a rare entity [6]. A leukemic phase of lymphoma is more commonly associated with mantle cell, follicular, and anaplastic lymphomas [1, 7, 8].
| Case Report | ▴Top |
A 66-year-old Caucasian female with a past medical history of exercise-induced pulmonary hypertension, obstructive sleep apnea, and asthma, presented with a diffuse, non-pruritic, purple rash of the bilateral lower extremities of 1 week duration. The rash was accompanied by one episode of fever and low back pain. The patient endorsed weight gain and did not report night sweats prior to presentation. Physical exam revealed a healthy-appearing, pale woman with non-blanching, pink and purple papules over both lower extremities, and one indurated, firm, pink and brown plaque over the left medial malleolus (Fig. 1).
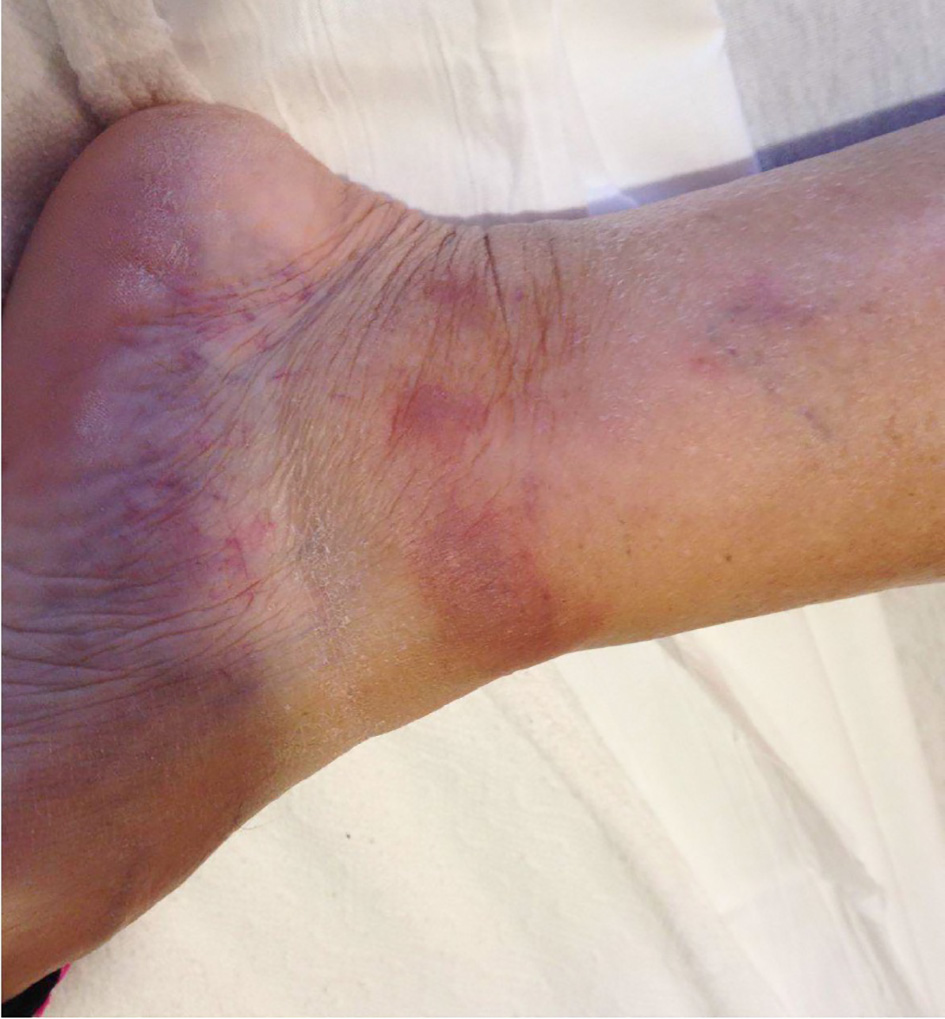 Click for large image | Figure 1. Rash over left medial malleolus seen on initial presentation. |
Laboratory studies revealed a leukocytosis with a total white blood cell (WBC) count of 46.6 × 109/L (28% polymorphonuclear cells, 13% band forms, 16% lymphocytes, and 34% atypical lymphoid cells (Fig. 2a)), lactate dehydrogenase > 3,600 IU/L, and uric acid 15.2 mg/dL.
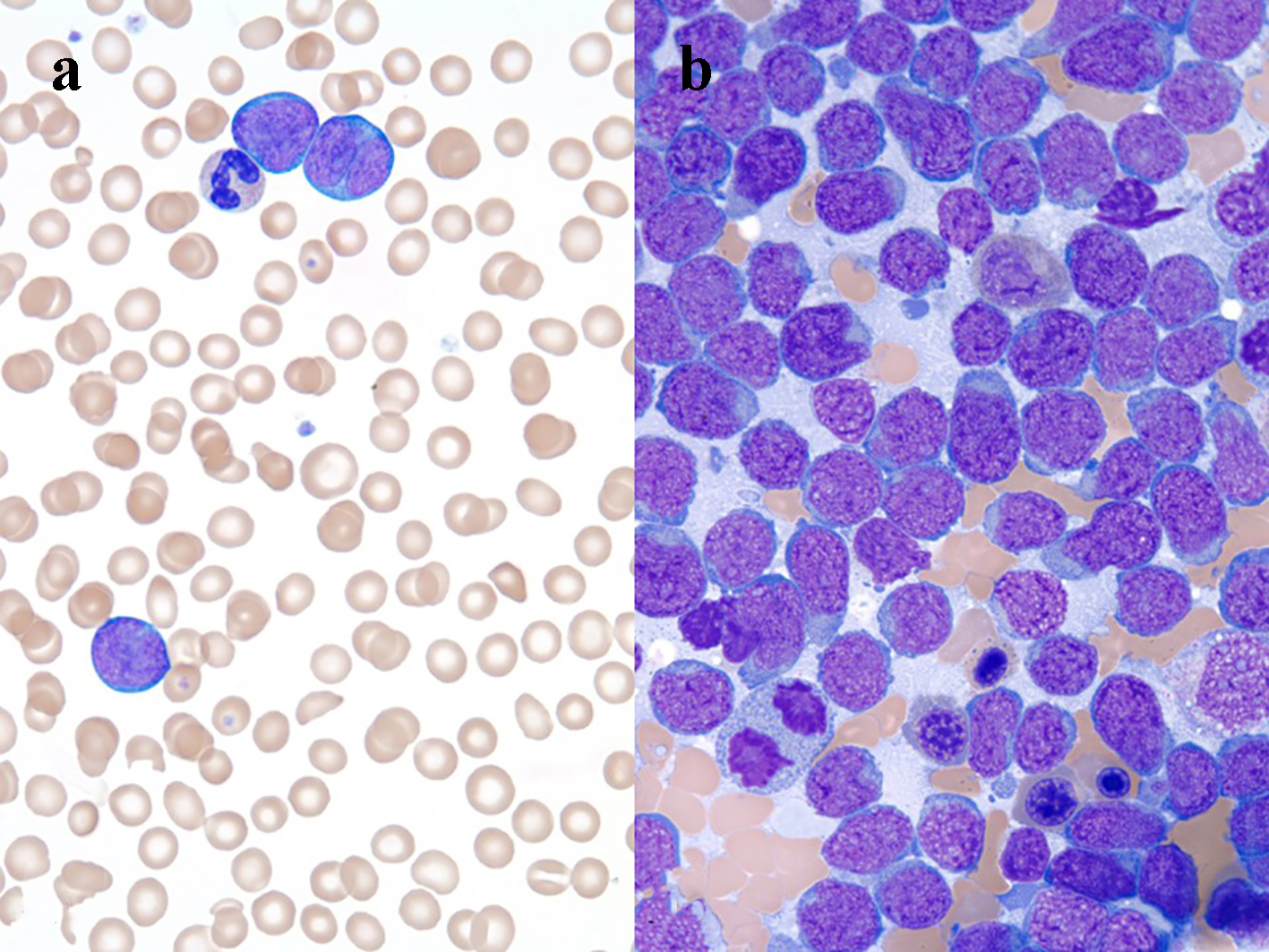 Click for large image | Figure 2. (a) Three large lymphoma cells and a neutrophil, peripheral blood smear, Wright’s stain, immersion oil, objective × 100. (b) Many lymphoma cells, bone marrow aspirate smear Wright’s stain, immersion oil, objective × 100. |
Radiographic studies of the chest, abdomen, and pelvis revealed minimally prominent mesenteric lymph nodes, which were not reported as pathologically enlarged, with no other mass or potential primary lesion identified.
Flow cytometry of peripheral blood identified 44% neoplastic B-lymphoid cells expressing CD19, CD20, CD10, and CD38. Analysis of bone marrow aspirate (Fig. 2b) and biopsy was remarkable for a hypercellular marrow with 80-90% blast-like, surface-IgG-positive B-lymphoid cells, which tested positive for MUM1, CD10, and bcl2. Ten percent of the abnormal population was c-myc-positive. The abnormal neoplastic B-lymphoid population was negative for cyclin D1, CD34, and TdT. FISH studies detected the presence of a t(14;18) translocation, IGH-BCL2 fusion, and deletion of CDKN2A and MLL. A c-myc rearrangement was not detected. Cutaneous punch biopsy (Fig. 3a) of the right medial malleolus showed a dense infiltration of the subcutaneous fat and dermis by CD20 (Fig. 3b) with only a small subset of admixed CD3-positive T cells noted (Fig. 3c). The B-lymphoid population was uniformly PAX5, MUM-1 (Fig. 3d), CD10 (Fig. 3e), bcl6 and CD31-positive and had a high proliferation rate (almost 100%) as highlighted by their immunoreactivity to the MIB-1 antibody (Fig. 3f). The neoplastic lymphoid population was negative for TdT, CD34, CD5, CD56, CD138, and EBV latent membrane protein antibody. Together, the findings were interpreted to be most consistent with a leukemic-phase DLBCL. Due to the associated co-expression of MUM1 and CD10, this lymphoma could not be further classified based on the Hans algorithm [9].
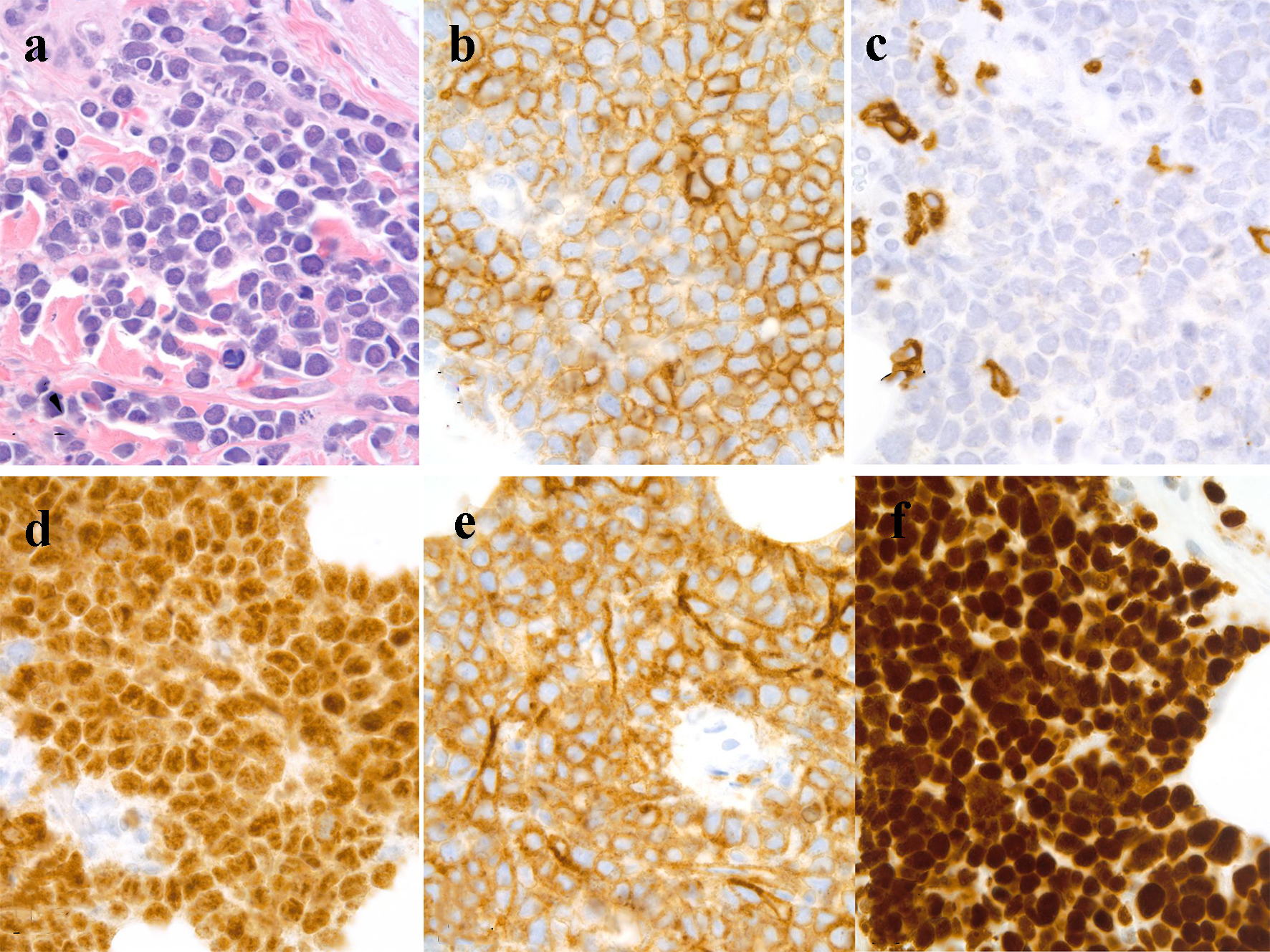 Click for large image | Figure 3. Skin, punch biopsy: (a) dense intradermal infiltrate of lymphoma cells, hematoxylin and eosin stain, immersion oil, objective × 100; (b) CD20-positive lymphoma cells, immersion oil, objective × 100; (c) a few CD3-positive T cells, immersion oil, objective × 100; (d) MUM1-positive lymphoma cells, immersion oil, objective × 100; (e) CD10-positive lymphoma cells, immersion oil, objective × 100; (f) MIB-1-positivity in lymphoma, immersion oil, objective × 100. |
Upon presentation, treatment was initiated with rasburicase and intravenous fluids for tumor lysis syndrome. Reports have shown that DLBCL with CDKN2A deletion has poor outcomes with standard R-CHOP therapy, so anti-neoplastic therapy was initiated with DA-R-EPOCH [10-12]. The patient subsequently sought care in another institution and continued treatment with R-CHOP and intrathecal methotrexate (MTX). The patient’s rash and leukocytosis resolved after the first cycle of DA-R-EPOCH. After the third cycle of R-CHOP and MTX, the patient presented to our emergency department with febrile neutropenia and mucositis, and was found to have a methotrexate level of 0.19 µmol/L. She ultimately died due to complications from severe sepsis. In this particular case, only pallor and rash, which is a non-descript finding, was seen on initial presentation. This is the first report of leukemic-phase DLBCL with its initial manifestation as a bilateral lower extremity infiltrative skin rash.
| Discussion | ▴Top |
NHL is the seventh most common cancer in the United States. In 2016, there was an estimated 73,000 new cases of NHL [13]. Of these cases, approximately 20-30% were DLBCL [3, 5, 14, 15]. Median age of diagnosis is 66, with a median age of death around 76 years old [3, 13]. Approximately 60% of patients present with a rapidly-enlarging lymph node in the neck, torso, or axillary region, although 40% of patients have extranodal disease [3]. Common extra-nodal sites are the gastrointestinal tract and stomach [3]. One-third of patients present with B symptoms, one-half present with elevated LDH, and bone marrow involvement ranges from 15% to 30% at initial presentation [3, 16, 17]. We present a case of leukemic-phase DLBCL in a Caucasian female with a rash and without B symptoms. While extra-nodal involvement is relatively common in DLBCL, malignant cells circulating in the peripheral blood are rare. The leukemic-phase of DLBCL is hypothesized to be from variable expression of adhesion molecules that result in lymphoma cell migration into the blood stream [18].
In a study by Muringampurath et al, 29 patients were identified to have leukemic-phase DLBCL with a median age of presentation of 48 years old. All patients had extra-nodal extramedullary disease, 100% had bone marrow involvement, 62% had spleen involvement, 41% had disease in the pleura, 21% reported to have infiltrative liver involvement, 7% had bowel involvement, and 14% had cerebrospinal fluid involvement [15].
DLBCLs are a malignant transformation of mature B cells. They can develop as a transformation of other types of NHL, follicular and CLL being the best studied, or can develop de novo. Immunophenotypically, DLBCLs are remarkable for positive B-cell lineage markers, CD19 CD20, CD22, CD79a, and CD45. These terminally differentiated B cells are further classified into germinal center B-cell (GCB) or activated-B-cell (ABC) subtypes by CD10, BCL6, and MUM-1 biomarker positivity. Twenty-five to 80% of DLBCLs express BCL-2, and 70% express Bcl-6. Thirty to 60% express CD10, and 35-65% express MUM1 [19, 20]. Simultaneous deletion of CDKN2A and gain of BCL2 have been reported in 35-65% of DLBCL cases [10, 19]. Ki67 (proliferation fraction of cells) is commonly > 90% [15, 20].
There are no specific genetic abnormalities that are diagnostic for DLBCL, but genetics to provide prognostic information. Bcl-6 over-expression is seen in 20-40% of DLBCL cases and is a common oncogenic mechanism [1, 19]. T(14;18) when present, can lead to suspicions of follicular transformation. However, it has been shown to be in 20-30% of de novo DLBCL cases [20, 21]. Most notably, CDKN2A mutations are currently under investigation.
CDKN2A tumor suppressor region can be found on the short arm of chromosome 9p2. Deletion of this segment has been seen in leukemias, melanomas, and bladder cancer. It has also been noted to be deleted or hyper-methylated in 25-50% of DLBCLs [22]. CDKN2A gene encodes three transcript variants, each that differs by their first exon. Two alternatively spliced variants encode distinct proteins that function to inhibit CDK4 kinase. p16(INK4a) attaches to CDK4 and CDK6. CDK4 and CDK6 stimulate the cell to continue through the cell cycle. p14(ARF) protein prevents degradation of p53. The other CDKN2A transcript contains an alternative open reading frame that acts as a stabilizer of p53, by interacting with and sequestering the E3-ubiquitin protein ligase MDM2, which degrades p53 [23]. CDKN2A also encodes a protein, p16, which interacts with cyclin D and the cell-cycle-dependent kinase CDK4 or 6, which inhibits the phosphorylation of retinoblastoma protein (pRB) [24]. Cyclin D and RB1 are oncogenes or tumor suppressor genes, respectively. There is an inverse correlation between inactivation of CDKN2A and inactivation of RB1 or over-expression of CDK2 [25]. The GELA study showed a loss of CDKN2A was associated with shorter survival after R-CHOP, independent of International Prognostic Score (IPI) and cell of origin. Of the 107 patients in the R-CHOP arm, 5-year OS and EFS were significantly worse, with a hazard ratio of 3.19 and 2.98, respectively. It was also seen that 52% of patients with CDKN2A deletion had gain of BCL2 [10].
In 1993, the IPI was established as the best predictor of outcomes, compared to the Ann Arbor classifications system. Factors indicative of poor outcomes include age > 60 years old, stage III/IV disease, elevated LDH concentration, performance status of 2 or greater, and number of extranodal sites. CD30 has been shown to confer a good prognosis, whereas CD5 and a high Ki-67 portend a poor prognosis [26]. High-risk DLBCL patients have a 5-year survival closer to 40% compared to lower-risk DLBCL patients, who have a reported median 5-year survival of 59% [1, 2, 15, 27]. t(14;18), BCL2 over-expression, and CDKN2A methylation or deletion have been shown to predict poor outcomes [27, 28]. Jardin et al showed that CDKN2A loss was associated with significantly shorter OS, defined as time from first chemotherapy to death from any cause, and EFS, defined as time between first chemotherapy cycle disease progression, relapse, or death from any cause, compared to no CDKN2A loss in the DLBCL population, with a hazard ratio of 2.72 (95% CI: 1.54 - 4.81, P < 0.001) and 2.31 (95% CI: 1.42 - 2.78, P < 0.001), respectively [10].
Survival for patients with DLBCL has improved significantly with the inclusion of anthracyclines and anti-CD20 agents in treatment. Since R-CHOP was introduced, 5-year OS has improved from 0% to 59-79% [29-31]. However, 40-50% of patients would experience primary treatment failure or relapse [27]. Thus, dose-adjusted R-EPOCH was introduced for patients with intermediate to high risk DLBCL, with a complete response (CR) rate of 86% with a 5-year EFS of 59% [27]. Garcia-Suarez et al showed that those patients whose tumors over-expressed BCL-2 benefited the most from the addition of rituximab to the EPOCH backbone. This finding was confirmed by Wilson et al in two phase II studies [11, 32]. Garcia-Suarez et al explored the use of DA-EPOCH-R in MLL- and CDKN2A-positive DLBCL and found it to be superior to R-CHOP, independent of cell origin or IPI score. With DA-EPOCH-R, patients achieved 83.8% CR, 68% EFS, and 75% OS at follow-up at 12 months [27]. A recent prospective study published by the Anderson showed that patients with MLL- and BCl2-positive DLBCL benefited from DA-EPOCH-R. Patients achieved 74% CR, 65% PFS, and 86% OS at follow-up at 12 months [33].
CNS involvement at the time of DLBCL diagnosis is uncommon. However, with disease in leukemic phase, 14% of patients were found to have brain involvement at diagnosis. Within a year of diagnosis, CNS relapse is seen in approximately 5% of all DLBCL patients, treated with rituximab [34, 35]. This is associated with a median survival of approximately 2 - 5 months. Whether intrathecal prophylaxis is warranted at initial diagnosis is still under debate [15]. Garcia-Suarez et al enrolled 20 patients with untreated, high-risk DLBCL (IPI score 2 - 3) in a study where they would receive eight cycles of DA-EDOCH14-R every 2 weeks, instead of the traditional every 3 weeks, without CNS prophylaxis. Three-year PFS and OS were both as high as 95%, with only one patient developing CNS recurrence [14]. It has been proposed that rapid tumor reduction in itself is adequate CNS prophylaxis given good CNS penetration of rituximab [14, 34]. Even with rituximab use, Zahid et al recommended CNS prophylaxis for those at high risk of CNS recurrence. Highest risk factors include high IPI score, high LDH, and more than one extra-nodal site (especially breast and testes) HIV positivity, and double-hit lymphomas. However, superiority of a single approach to CNS prophylaxis, intrathecal vs. systemic methotrexate, intrathecal methotrexate, and intravenous cytarabine has not been demonstrated [34].
Conclusion
DLBCL is a heterogeneous group of diseases, typically presenting with LAD, B symptoms, and fevers. We present the first case of leukemic-phase DLBCL manifesting solely as a lower extremity rash. DLBCL has a cure rate estimated at 50-60% [27]. With the advent of new biomarkers, therapies may be tailored to improve outcomes in different disease subtypes.
Author Note
Prior to generation of this manuscript, the patient provided written consent authorizing the publication of this report.
Conflicts of Interest
None.
| References | ▴Top |
- Pires PP, Kanegae MY, Rays J, Catania M, Lima FR, Noronha TR, Abdo AN, et al. Diffuse large B-cell lymphoma presenting in the leukemic phase. Autops Case Rep. 2016;6(1):41-45.
doi pubmed - Loong F, Chan AC, Ho BC, Chau YP, Lee HY, Cheuk W, Yuen WK, et al. Diffuse large B-cell lymphoma associated with chronic inflammation as an incidental finding and new clinical scenarios. Mod Pathol. 2010;23(4):493-501.
doi pubmed - Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132(1):118-124.
pubmed - Control, U.f.I.C. Diffuse Large B-Cell Lymphoma. 2014 Review of Cancer Medicines on the WHO list of Essential Medicines. 2014.
- Oluwole OO, Zic JA, Douds JJ, Ann Thompson M, Greer JP. Cutaneous manifestations and management of hematologic neoplasms. Semin Oncol. 2016;43(3):370-383.
doi pubmed - Hazarika B. Diffuse large B-cell lymphoma in leukemic phase. Blood. 2014;124(13):2159.
doi pubmed - Nogai H, Dorken B, Lenz G. Pathogenesis of non-Hodgkin's lymphoma. J Clin Oncol. 2011;29(14):1803-1811.
doi pubmed - De Paepe P, De Wolf-Peeters C. Diffuse large B-cell lymphoma: a heterogeneous group of non-Hodgkin lymphomas comprising several distinct clinicopathological entities. Leukemia. 2007;21(1):37-43.
doi pubmed - Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282.
doi pubmed - Jardin F, Jais JP, Molina TJ, Parmentier F, Picquenot JM, Ruminy P, Tilly H, et al. Diffuse large B-cell lymphomas with CDKN2A deletion have a distinct gene expression signature and a poor prognosis under R-CHOP treatment: a GELA study. Blood. 2010;116(7):1092-1104.
doi pubmed - Wilson WH, Jung SH, Porcu P, Hurd D, Johnson J, Martin SE, Czuczman M, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758-765.
doi pubmed - Wilson W, Ho J, Pitcher B, Hsi E, Friedberg J, Cheson B, Bartlett N, et al. Phase III randomized study of R-CHOP Versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-Cell Lymphoma: CALGB/Alliance 50303. Blood: ASH 58th Annual Meeting & Exposition, 2016.
- National Cancer Institute: Surveillence E, and End Results Program, SEER Stat Fact Sheets: Non-Hodgkin Lymphoma, in Cancer Statistics. 2016, National Cancer Institute's Division of Cancer Control and Population Sciences.
- Garcia-Suarez J, Flores E, Callejas M, Arribas I, Gil-Fernandez JJ, Olmedilla G, Curto N, et al. Two-weekly dose-adjusted (DA)-EPOCH-like chemotherapy with high-dose dexamethasone plus rituximab (DA-EDOCH14-R) in poor-prognostic untreated diffuse large B-cell lymphoma. Br J Haematol. 2013;160(4):510-514.
doi pubmed - Muringampurath-John D, Jaye DL, Flowers CR, Saxe D, Chen Z, Lechowicz MJ, Weisenburger DD, et al. Characteristics and outcomes of diffuse large B-cell lymphoma presenting in leukaemic phase. Br J Haematol. 2012;158(5):608-614.
doi pubmed - Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780-2795.
doi pubmed - A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909-3918.
pubmed - Bain BJ, Catovsky D. The leukaemic phase of non-Hodgkin's lymphoma. J Clin Pathol. 1995;48(3):189-193.
doi - Skinnider BF, Horsman DE, Dupuis B, Gascoyne RD. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol. 1999;30(7):803-808.
doi - Huang JZ, Sanger WG, Greiner TC, Staudt LM, Weisenburger DD, Pickering DL, Lynch JC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99(7):2285-2290.
doi pubmed - Aamot HV, Torlakovic EE, Eide MB, Holte H, Heim S. Non-Hodgkin lymphoma with t(14;18): clonal evolution patterns and cytogenetic-pathologic-clinical correlations. J Cancer Res Clin Oncol. 2007;133(7):455-470.
doi pubmed - Guney S, Jardin F, Bertrand P, Mareschal S, Parmentier F, Picquenot JM, Tilly H, et al. Several mechanisms lead to the inactivation of the CDKN2A (P16), P14ARF, or CDKN2B (P15) genes in the GCB and ABC molecular DLBCL subtypes. Genes Chromosomes Cancer. 2012;51(9):858-867.
doi pubmed - CDKN2A cyclin dependent kinase inhibitor 2A [Homo sapiens (human)]. Gene 2016 10/2/2016 [cited 2016 105/2016]; Gene ID: 1029.
- Dreyling MH, Roulston D, Bohlander SK, Vardiman J, Olopade OI. Codeletion of CDKN2 and MTAP genes in a subset of non-Hodgkin's lymphoma may be associated with histologic transformation from low-grade to diffuse large-cell lymphoma. Genes Chromosomes Cancer. 1998;22(1):72-78.
doi - Bodoor K, Matalka I, Hayajneh R, Haddad Y, Gharaibeh W. Evaluation of BCL-6, CD10, CD138 and MUM-1 expression in diffuse large B-cell lymphoma patients: CD138 is a marker of poor prognosis. Asian Pac J Cancer Prev. 2012;13(7):3037-3046.
doi pubmed - Doggett RS, Wood GS, Horning S, Levy R, Dorfman RF, Bindl J, Warnke RA. The immunologic characterization of 95 nodal and extranodal diffuse large cell lymphomas in 89 patients. Am J Pathol. 1984;115(2):245-252.
pubmed - Garcia-Suarez J, Banas H, Arribas I, De Miguel D, Pascual T, Burgaleta C. Dose-adjusted EPOCH plus rituximab is an effective regimen in patients with poor-prognostic untreated diffuse large B-cell lymphoma: results from a prospective observational study. Br J Haematol. 2007;136(2):276-285.
doi pubmed - Barrans SL, Evans PA, O'Connor SJ, Kendall SJ, Owen RG, Haynes AP, Morgan GJ, et al. The t(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin Cancer Res. 2003;9(6):2133-2139.
pubmed - Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013-1022.
doi - Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5):941-952, ix.
doi pubmed - Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242.
doi pubmed - Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, Raffeld M, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717-2724.
doi pubmed - Sathyanarayanan V, Oki Y, Issa AF, Ahmed MA, Noorani M, Fanal M, Hagemeister F, et al. High risk diffuse large b cell lymphoma: a comparison of aggressive subtypes treated with dose adjusted chemotherapy-the university of Texas MD anderson experience. Blood ASH 58th Annual Meeting & Exposition. 2016.
- Zahid MF, Khan N, Hashmi SK, Kizilbash SH, Barta SK. Central nervous system prophylaxis in diffuse large B-cell lymphoma. Eur J Haematol. 2016;97(2):108-120.
doi pubmed - Guirguis HR, Cheung MC, Mahrous M, Piliotis E, Berinstein N, Imrie KR, Zhang L, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol. 2012;159(1):39-49.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


