| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 1, Number 4-5, October 2012, pages 77-88
Oxidative Stress in Patients With Early Stage Chronic Lymphocytic Leukemia, Assessment and Correlation With Prognostic Factors
Xavier Ortina, d, Montserrat Giraltb, Marta Romeub, d, Marylene Lejeunec, M. Rosa Noguesb, Vanessa Sanchez-Martosb, Marta Rodriguez-Luacesa, Teresa Sansa, Llorenç Fonta
aHematology Department, Hospital de Tortosa Verge de la Cinta, Tortosa, Spain
bFarmacology Department, Unitat de Ciencies Mediques Basiques, Facultat de Medicina i Ciencies de la Salut, Universitat Rovira i Virgili, Reus, Spain
cMolecular Biology and Research Section, Hospital de Tortosa Verge de la Cinta, IISPV, URV, Tortosa, Spain
dCorresponding authors: Xavier Ortin, Hematology Department, Hospital de Tortosa Verge de la Cinta. C/Esplanetes 14, 43500 Tortosa, Spain. Marta Romeu, Unitat de Farmacologia, Departament de Ciencies Mediques Basiques, Facultat de Medicina i Ciencies de la Salut, Universitat Rovira i Virgili, C/Sant Llorenc 21, 43201-Reus, Spain
Manuscript accepted for publication October 4, 2012
Short title: Oxidative Stress in Lymphocytic Leukemia
doi: https://doi.org/10.4021/jh34w
| Abstract | ▴Top |
Background: The heterogeneous clinical outcome that characterizes chronic lymphocytic leukemia (CLL) can be predicted by a serie of clinical prognosis factors. However, the existence of a significant subset of patients with early stage CLL makes crucial the need of more accurate prognostic markers allowing the possibility of early treatment of these patients. Although high reactive oxygen species (ROS) levels have been seen to play a role in CLL, discrepancies in the information compiled about oxidative stress (OS) in these patients have been reported.
Methods: In this study, we evaluate the overall OS status, through a recently defined score of oxidative stress (SOS), of 37 early stage CLL patients and we compare SOS with well-known prognosis factors and with a matched control group of healthy individuals.
Results: We have observed an imbalance in the antioxidant/prooxidant equilibrium in early stage CLL patients with a significant higher SOS than that of the healthy control group (0.00 ± 0.53 vs. 2.97 ± 1.13; P < 0.05). Most of the patients who exhibit 3 - 4 adverse prognosis factors also present higher values of most of the OS biomarkers and SOS.
Conclusions: These data strongly suggest that the combination strategy of OS biomarkers with already known prognosis factor may have potential clinical applications in early stage CLL patients.
Keywords: Chronic lymphocytic leukemia; Prognosis; Oxidative stress; Antioxidant enzymes; Lipid peroxidation
| Introduction | ▴Top |
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in Western countries. The clinical course is remarkably variable; some patients live out their life not needing treatment and die of unrelated causes, whereas others have rapidly progressive disease requiring treatment within months of diagnosis and succumb to their disease within 2 to 3 years. Clinical features in Rai and Binet staging systems [1, 2] still remain the most important prognostic indicators in CLL. However, these clinical staging systems, developed in the late 1970s, have lost some of their usefulness, since most patients are now being diagnosed at an early stage, reflecting a broader use of routine blood evaluations. As a logical consequence, there is still a need for a simple and reliable method of risk stratification suitable for all patients with CLL. Furthermore, for each stage there is still heterogeneity, limiting utility in predicting survival.
In the understanding of the biology of CLL, a great number of new prognostic factors have been identified in addition to common factors used in clinical staging [3]. Different studies have described the predictive value of these parameters with regard to overall survival, disease progression and response to therapy. Other parameters are the presence of chromosome abnormalities such as 17p deletion and 11q deletion, elevated serum levels of β-2 microglobulin (β-2M), thymidine kinase, soluble CD23, unmutated immunoglobulin heavy chain variable gene (IgVH) status of CLL cells, and expression of ZAP-70 and CD38 by leukemia cells have been correlated with poor prognosis [4-9]. In addition to the fact that in isolation, each of these prognostic factors has limited utility in predicting overall survival, it is not yet defined how these factors should be used in the management of CLL patients [10-12]. The incorporation into daily practice of these markers must be standardized and validated in large prospective trials.
Reactive oxygen species (ROS) and oxygen free radicals (OFR) are both generated during physiological and pathological cellular metabolic activities playing a dual role in biological systems. On the other hand, they may be harmful, since high concentrations of ROS can lead to cellular damage including lipid peroxidation, DNA adduct formation, protein oxidation and enzyme inactivation, which can all ultimately lead to cell death [13-15]. But on the other hand they may be beneficial to living systems since a moderate level of intracellular ROS is thought to be important to maintain appropriate redox balance and to stimulate cellular proliferation [16]. To neutralize OFR, living organisms have defence mechanisms which are able to quench OFR, scavenge damaged molecules and repair molecular injuries. Among these defence mechanisms, there are no enzymatic molecules such as reduced glutathione (GSH), ascorbic acid, tocopherols, β-carotene and ubiquinone, and also several enzymatic systems such as catalase (CAT), superoxide dismutase (SOD), thioredoxin reductase, GSH-reductase (GR), GSH-peroxidase (GPx) and GSH S-trasnferase (GST) [13, 17, 18].
Extensive evidence has shown that disturbances of oxidative stress (OS) metabolism are a common feature of transformed tumor cells. Malignant cells such as human leukemic HL-60 cells and primary human leukemic cells (lymphocytes from CLL patients) have been demonstrated to produce more superoxide anions [19]. This intrinsic OS may explain the therapeutic selectivity of chemotherapeutic agents. However, toxicity caused by chemotherapeutic agents, such as cyclophosphamide and ifosfamide that induce urotoxicity, can be ameliorated by antioxidants, such as the chemoprotectant mesna, a thiol antioxidant [20]. There are few studies evaluating plasma and erythrocytes antioxidant status in CLL patients. Information about the activities of antioxidant enzymes is generally partial and discrepancies have been reported in relation to changes of enzymatic activities such as SOD and CAT activities in CLL patients [12, 21]. In the same way, the role of the higher concentration of GSH observed in lymphocytes in determining a prognosis in CLL subjects remains conflicting [13]. So, information about OS biomarkers in early stage CLL patients has not been yet well defined and it is possible that these may present imbalance between the mechanisms that generate ROS and the antioxidant defense mechanisms in favor of the latter, and thus show a higher OS levels than healthy population.
In this study, we have studied the OS profile in a group of CLL patients diagnosed of early stage and in a matched control group through the determination of the main enzymatic antioxidant defenses (SOD, CAT, GR and GPx) and the determination of the lipidic peroxidation products such as the thiobarbituric acid reactive substances (TBARS) and the reduced and oxidized glutathione (GSH, GSSG). Moreover we set the global antioxidant capacity and also a model of overall score to assess the degree of oxidative stress (SOS) in CLL patients compared with a control population. Finally, we assessed whether there were differences in markers of oxidative distress in our study population in relation to different recognized prognostic factors analyzed.
| Design and Methods | ▴Top |
Patients and control groups
Thirty-seven untreated early stage CLL patients were included in the study. All patients were enrolled during a period of 10 months. The diagnoses were based on the International Worshop on CLL (IWCLL) criteria [22] and the clinical stage was defined according to the systems defined by Rai and Binet [1, 2]. The inclusion criteria of these patients were: to have a medical and laboratory tracking in the Department of Hematology of the” Hospital de Tortosa Verge de la Cinta”, to have a A/0-1 stage as Rai and Binet staging system, not having chronic associated diseases (diabetis, renal insufficiency, hepatopatia and other neoplasies), being without treatment (for CLL, diabetis or hypertension), without signs of disease progression during the follow-up period (TDL > 12 months) and without toxic habits (tobacco, alcohol). Thirthy-seven age and sex-matched healthy subjects were included in this study as control group. The samples were obtained from the Department of Endocrinology of the Institute Dexeus and from healthy volunteers who attended the Department of Pharmacology of the “University Rovira i Virgili”. These healthy individuals were enrolled on the basis of specific inclusion criteria: individuals without pathology and without relevant treatment such as an antioxidant at the moment of the analysis. All CLL patients and healthy control individuals gave written informed consent. The local Ethics Committees from the “Hospital de Tortosa Verge de la Cinta” and the “University Rovira i Virgili” approved the study.
Clinical records of patients included the principal variables that were already known to be of prognostic relevance: lactacte dehydrogenase (LDH), β-2M, immunophenotypic score (CD5, CD23, FMC7, sIg, CD22) [23], presence of CD38 and ZAP-70 detected by flow cytometry, karyotipe by conventional cytogenetic (del 13, +12, del 11, del 17), presence of lymphadenopathy, liver or spleen enlargement by CT scan, and determination of lymphocyte morphology and lymphocytary doubling time (LDT).
In the two groups of the study, we determined the following markers of OS: SOD, CAT, GPx, GR, GSH, GSSG, TBARS and global antioxidant capacity through the ORAC method. The determination of SOS was based on the analysis of these all these biomarkers.
Sample collection
10 ml of blood samples were taken from patients and control individuals in vaccutainer EDTA and Li-Heparin tubes, kept under refrigeration and processed no later than six hours after collection. The plasma and the erythrocytes were separated by centrifugation at 850 ×g at 4 °C during 15 min. Plasma from EDTA samples was directly aliquoted and stored at -20 °C for further determination of TBARS content. Erythrocytes from EDTA samples were washed twice with physiologic serum, centrifuged at 1,900 ×g for 5 min at 4 °C and stored at -20 °C so that SOD, GPx, GR and CAT activities could be determined. Plasma from heparinized samples was divided in two aliquots. The first aliquot was stored directly at -20 °C forfurther determination of GSH and GSSH. The second aliquot was deproteinized with trichloroacetic acid at a final concentration of 10% (TCA 70%) and stored at -20 °C for further determination of plasma GSH and GSSG. Erythrocytes from heparinized samples were washed twice with physiologic serum (1,300 ×g or 5 min at 4 °C) and then lysed with a phosphate buffer at pH 6.25. Hemolyzed samples were treated with cold trichloroacetic acid at a final concentration of 10% (TCA 70%) and stored at -20 °C for further analysis of erythrocyte GSH and GSSG and TBARS.
Determination of antioxidant enzymes activities
For the determination of SOD activity, washed erythrocytes from EDTA samples were frozen and thawed twice and then lysed with 5 volumes of distilled water. Haemoglobin was extracted with ethanol:chloroform (6.25:3.75) and after centrifugation at 1,900 ×g during 5 min at 4 °C. SOD was recovered in the supernatant phase. The SOD activity was measured by the Misra and Fridovich method [24] based on the autooxidation of epinephrine and expressed as units/g of haemoglobin (one unit, U = the amount of sample that inhibits the transformation of epinephrine into adrenochrome by 50 %). The reaction was monitored at 480 nm (Espectrometer Perkin Elmer Lambda 25).
For the determination of CAT, GR and GPx activities, washed erythrocytes were lysed with 10 volumes of cold bidistilled water. CAT activity was determined in the hemolyzed samples by the Cohen method [25], which monitored the rate at which hydrogen peroxide (15 mM) disappeared at 240 nm (Espectrometer Perkin Elmer Lambda 25). This was expressed in mmol of hydrogen peroxide transformed/min/g of haemoglobin. GR and GPx activities were determined by the Wheeler method [26], which monitors the rate at which NADP+ or NADPH disappear at 340 nm (Espectrometer Perkin Elmer Lambda 25). GR and GPx were expressed in µmol of NADPH transformed/min/g of haemoglobin.
Determination of lipids and peptides peroxidation products
GSSG is considered as a peroxidation product of peptides. The presence of GSSH and GSH in untreated and deproteinized plasma as well as in hemolyzed erythrocytes from heparinized samples was determined by fluorimetry with the Hissin and Hilf method [27] at 350 nm (lex) and 420 nm (lem) wavelengths (Espectrofluorometer Perkin Elmer LS55). The GSH and GSSH react with O-phtalaldialdehyde (OPT) as the fluorescent reagent. The calculated GSSG/GSH ratio is considered the redox value that best determines the antioxidant capacity of cells [28], and any increase suggests a strong prooxidant effect [29, 30].
TBARS were estimated according to the method of Buege and Aust [31] but using fluorescence at 515 nm (lex) and 548 nm (lem) wavelengths as described by Richard et al [32] (Espectrofluorometer Perkin Elmer LS55). Lipid peroxidation was measured as malondialdeyde (MDA) equivalents using trichloroacetic acid, thiobarbituric acid and hydrogen chloride. The increase in TBARS content was taken to indicate oxidative damage.
Determination of global antioxidant capacity
Global antioxidant capacity was measured trough the ORAC method [33]. The method is based on the the measurement of the antioxidant capacity to trap peroxil radicals induced by 2, 2’-azobis (2-amidinopropane) dihydrochloride at 37 °C. The fluorometric reaction with the 6-Hydroxy-2, 5, 7, 8-tetramethylchroman-2carbolxylic acid (trolox) was measured in a Fluorscan Ascent Analyzer (excitation 540 nm, emission 565 nm). The value area under the curve (AUC) permits to obtain the ORAC value as follow: ORAC = ((AUCsample - AUCcontrol) / (AUCtrolox - AUCcontrol)) × (molarity of trolox/molarity of the sample).
Determination of the Score of oxidative stress (SOS)
To evaluate the global SOS we have utilized a method previously described by Romeu et al [34], modified in function of the data obtained from the literature and from the statistical analyses of the current results from control group. In this method, each biomarker received a numerical value of 0, +1 or -1 depending on the value of the biomarker obtained during the analysis and the range of “standard” normal values established from the control group. As the result of the adaptation of the scoring method to the analysis of LLC patient and healthy controls included in the study, the values of the different biomarkers were scored as described in Table 1.
 Click to view | Table 1. Scoring Criteria for Each Biomarker to Derive the Score of Oxidative Stress (SOS) |
The SOS values of the control group were normally distributed around the mean which, by definition, was 0 point and represent an antioxidant-prooxidant equilibrium. A score of -1 was done for an antioxidant status (AS) and a score of +1 was done for an OS status. In these conditions, a score of -1 was done for high values of antioxidant enzymes (SOD, CAT, GPx, and GR) and antioxidant molecule GSH but also for low values of GSSH, GSSH/GSH ratio and TBARS in erythrocytes and in plasma. On the contrary, a score of +1 was assigned for low values of antioxidant enzymes and the antioxidant GSH but for high values of GSSH, GSSH/GSH ratio and TBARS. This new multi-biomarker score (SOS) was obtained using a simple mathematical formula (SUM function) linked to a Microsoft Excel® spreadsheet.
Although conservatory precautions were taken in order to minimize a possible oxidation of the samples during their manipulation [35, 36], plasmatic GSH, GSSG and TBARS biomarkers have not been considered for the determination of SOS. In the same way, ORAC has not been included in the SOS, since it represents a global measure of the antioxidant capacity.
Statistics
All the data were processed with the SPSS statistical package 13.0 (SPSS Inc, Chicago, IL). The different parameters were expressed as the mean and the standard deviation. The Kolmogorov-Smirnov test was performed to test the normality and the Fisher-Snedecor test to test the homogeneity the variances, 5th and 95th percentiles were obtained for all biomarkers of the healthy control samples and used as the lower and upper limits of normality for the population. Significant differences between the results obtained from patients and those from healthy controls were compared by one-way ANOVA, Student’s t-test, Mann-Whitney U test or Wilcoxon’s signed rank, as appropriate. When more than two groups were compared, the significant differences were assessed by Kruskal-Wallis (H-test) or Friedman tests, as appropriate. Correlation studies were assessed by Spearman’s Rank Order correlation (rho). Differences were considered significant when the probability was P < 0.05.
| Results | ▴Top |
Characteristics of the 37 CLL patients of the study
The 37 early stage CLL patients diagnosed within our geographic area were representative of a general population of early stages CLL. They present a general characteristic defined in this population, which is the advanced mean age, 75 ± 9.78 years (age extreme values ranging between 54 and 90 years) without a clear gender predominance (20 women, 17 men). As a comparison, the mean age of healthy volunteers was 72 ± 7.18 years. The median follow-up of the CLL patients was 76 months (from 20 to 271 months) and during this time none of the patients showed disease progression to more advanced stages.
Regarding the prognostic factors analyzed initially in our sample (Table 2), 18.91% of patients did not show any adverse prognostic factor (7/37), 64.86% had one or two adverse factors (24/37), and 16.21% had three or four adverse factors (6/37). The maximum number of four adverse prognostic factors was identified in only one patient.
 Click to view | Table 2. Characteristics of Known Prognostic Factors of the 37 CLL Patients Included in the Study |
Imbalance in the antioxidant/prooxidant equilibrium in early stage CLL patients
Figure 1 summarizes the values of OS biomarkers determined in erythrocytes and plasma of the CLL and the healthy control group. Since the control group comprises individuals who have not been exposed to the main exogenous factors of ROS production, the values of their biomarkers have been used as a reference. A significant higher degree of OS could be observed in CLL patients as compared to the healthy control group. All erythrocyte biomarkers were significantly different in CLL patients as compared to the values of the control group. The glutathione system in erythrocytes of early stage CLL patient was altered since these patients present a significant increase in GSSG and the ratio GSSG/GSH. Moreover, the significant decrease in the level of the antioxidant molecule GSH reveal a probably malfunctioning of the system of turnover of GSH in erythrocytes. In the same order, the antioxidant enzymes SOD, CAT, GPx and the GR were also significantly decreased in the patient group compared to the control group. The imbalance in the antioxidant/prooxidant equilibrium in early stage CLL patients is reflected by a significant decrease in the antioxidant enzymatic systems and also a significant decrease of the lipid peroxidation products TBARS in erythrocytes.
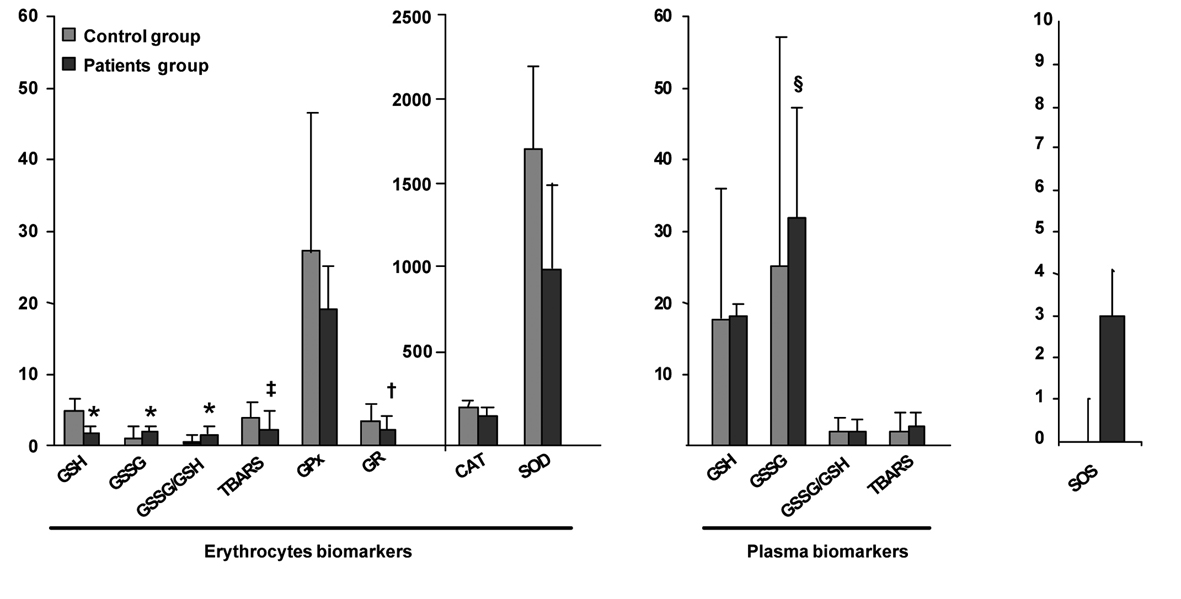 Click for large image | Figure 1. Comparison of OS biomarkers values of early stage CLL and healthy control groups. Results are presented as mean ± standard deviation (erythrocytes biomarkers GSH, GSSG, GSSG/GSH, CAT, GPx, SOD and plasma biomarkers GSSG, GSSG/GSH) or geometric mean ± antilog standard deviation (erythrocytes biomarkers TBARS, GR and plasma biomarkers GSH, TBARS) in the healthy control group (n = 37) and early stages of CLL patients group (n = 37). *Statistical differences between healthy control and early stages of CLL patients with a P value < 0.001, †with a P value = 0.001, ‡with a P value = 0.013, §with a P value = 0.016. |
Concerning the plasma biomarkers, we could observe that CLL patients present only significantly increased levels of GSSG as compared to the control group. No significant changes have been observed in the gluthatione system (GSH, P = 0.778; GSSG/GSH, P = 0.784) and the lipid peroxication products TBARS (P = 0.180).
SOS system in early stage CLL patients
The values obtained with our new multi-biomarker score clearly reflected the differences observed between the levels of the different biomarkers of CLL patients and the healthy controls (Fig. 1). Effectively, early stage CLL patients present a much higher SOS with almost three points OS of difference in comparison to the control group (P < 0.001). As shown in the graphical representation of the frequency distribution of the SOS of patients and controls groups (Fig. 2), healthy control individuals present a normal distribution centered on zero. However, the frequency distribution of the SOS on early stage CLL did not follow a normal distribution, since the score was displaced towards positive values, namely, OS.
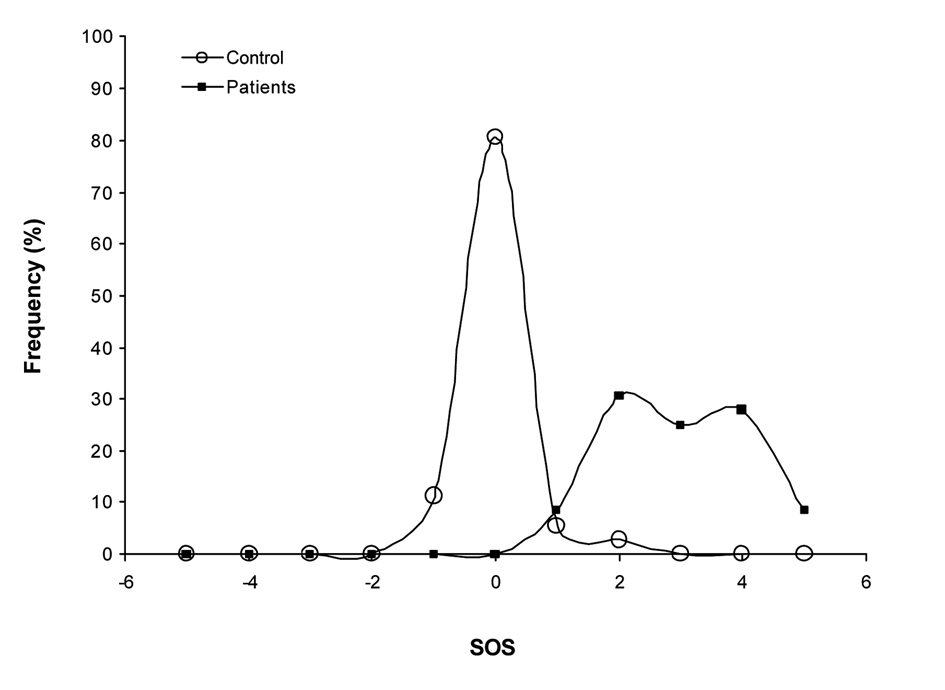 Click for large image | Figure 2. Frequency distribution of the SOS. Healthy control individuals present a normal distribution centered on zero. The frequency distribution of the SOS on early stage CLL did not follow a normal distribution, since the score was displaced towards positive values. |
Relationship between oxidative stress and prognostic factors in early stage CLL
Although we do not have an ORAC value of healthy controls, ORAC values of CLL patients have been correlated with their OS biomarkers. ORAC correlated negatively with the level of the antioxidant enzymes SOD and GPx and we found a positive correlation with the erythrocytes and plasma values of TBARS, GSSG and the plasmatic level of GSSG/GSH. Globaly, ORAC increases in the same manner as SOS. This seems to indicate that in parallel with the OS status, CLL patients present mechanisms of compensation that respond to the attack of the radicals.
The correlation of the different OS biomarkers with the already known prognostic markers was carried out in order to consider whether any of these biomarkers could also be included as prognostic factors of progression of the disease in the early stages of CLL. No significant differences could be observed in the values of the OS biomarkers in relation with the two immunophenotypic scores (5 vs. ≤ 4). However, a tendency to higher levels of erythrocytes and plasmatic TBARS as well as lower levels of the antioxidant enzymes SOD and GR could be observed in patients with adverse prognostic factors. In the same way, as observed in the Figure 3 A, B, no significant differences have been observed in the levels of OS biomarkers in relation to the prognostic CD38 and ZAP-70. Only an increase of 0.43 points of SOS has been observed in CD38 positive patients as compared to CD38 negative patients. Only one patient was ZAP-70 positive. For these patients the analysis of coefficients of variation of the different OS biomarkers indicated an imbalance in the antioxidant/prooxidant equilibrium (increase of plasmatic TBARS and GSSG, increase of erythrocyte TBARS, GSSG, GSSG/GSH and overall SOS as well as an impairment of the antioxidant enzymes GSH, SOD, CAT, GR and GPx). Considering the cytogenetic findings, only two patients presented adverse alterations in the karyotype (Fig. 3C). In these two patients a greater grade of OS could be observed as compared to patients with a normal karyotype or with favorable prognosis abnormalities. Contradictory results were obtained with the the LDH values since patients with adverse prognosis (LDH > 500U/L) presented favorable SOS punctuations. Regarding ß-2M (Fig. 3D), a tendency of higher SOS could be observed in the group of patients with an adverse prognosis (β-2M > 2.4 mg/L) without significance. Individually, the level of erythrocyte GSSG was significantly higher in patients with adverse prognosis.
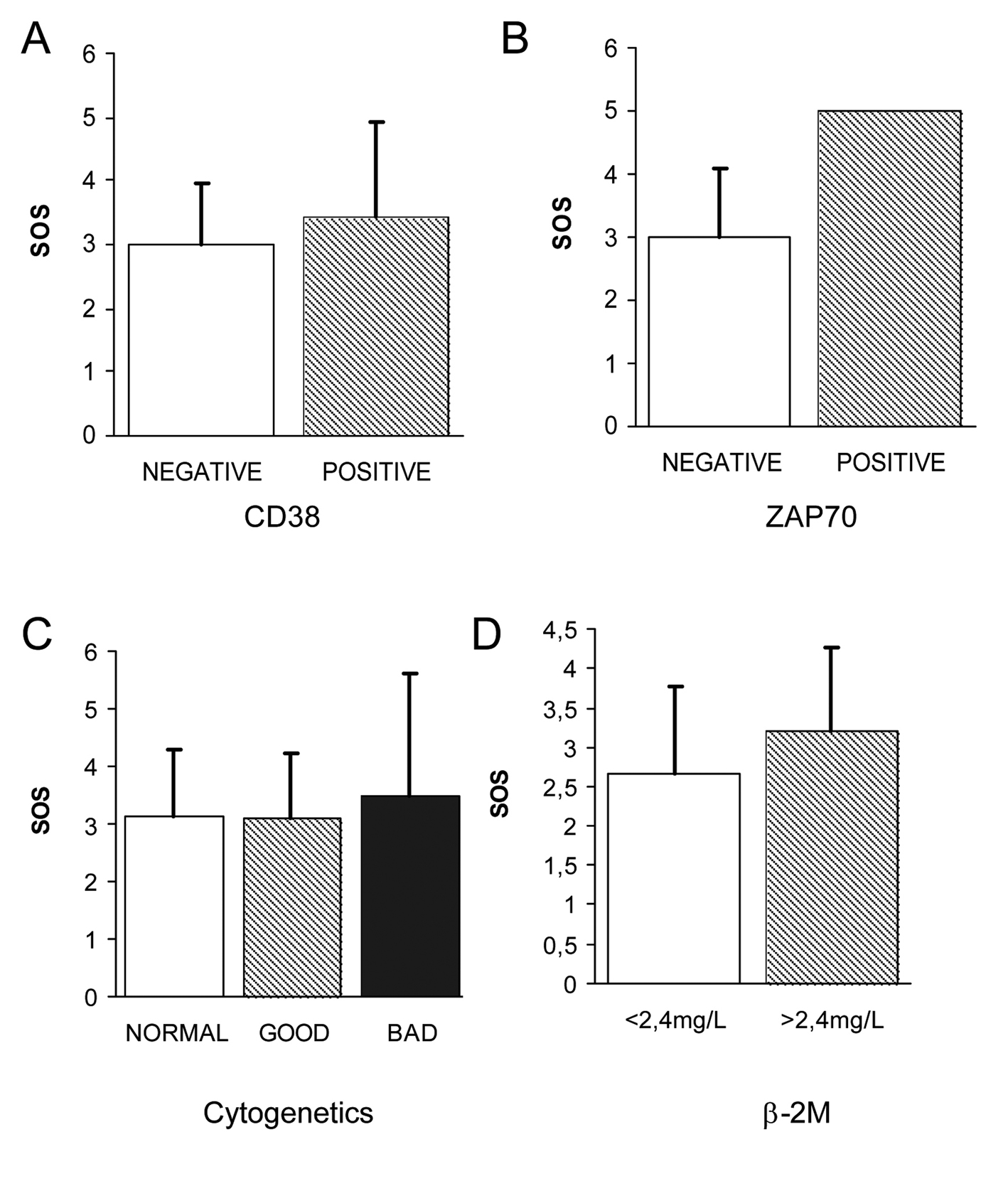 Click for large image | Figure 3. Correlation of OS biomarkers with evaluated prognostic of early stage CLL. (A) No significant differences could be observed in the levels of OS biomarkers CD38 (A), ZAP70 (B) and cytogenetic karyotipe (C) in relation to the prognostic. Regarding β-2M (D), a tendency of higher SOS could be observed in the group of patients with an adverse prognosis (β-2M > 2.4 mg/L) without significance. |
Since our sample size (37 samples) is not large enough to derive any really significant conclusions from each group of risk factors, we created a new variable related to the number of adverse prognostic factors (NAPF) derived from the all the prognostic factos evaluated in this study. The NAPF differentiates LLC patients who have no adverse prognostic factors, who have 1 - 2 of them and who have 3 - 4 of them. Then we have combined the different items of NAPF with the different SOS punctuations. The frequency distribution of different combinations showed a tendency to a more accurate classification of early stage LLC patients in 3 groups: a group of patients with a favorable prognosis (NAPF of 0 and SOS of 1), a group of patients with an adverse prognosis (NAPF of 3-4 and SOS of 5) and a group of patients with an uncertain prognosis (NAPF of 1-2 and SOS of 2-4). The group of patients with an SOS punctuation of 5 included three patients that present significant alterations in the values of GSH, GSSG, and GSSG/GSH from erythrocytes and CAT biomarkers. Only 2 of these three patients presented also alterations in the plasmatic TBARS values and GR (data not shown). Globally, the great majority of the biomarkers indicated a greater OS in the group of patients with adverse prognosis (Table 3).
 Click to view | Table 3. Values of OS Biomarkers in Function of the 3 Groups of NAPF |
| Discussion | ▴Top |
The effect of OS on poor health of individuals is unquestionable. Because of their reactive chemical properties, ROS may cause various types of tissue injury in a wide range of human diseases, and an excessive amount of ROS can lead to cell death by apoptosis or by necrosis [37, 38]. The ability of ROS to damage cellular components and cause cell death suggests a possibility to exploit this chemical property for killing cancer cells through a free radical-mediated mechanism. This strategy is of therapeutic relevance, because most cancer cells are active in metabolic production of ROS and are intrinsically under increased OS, and thus more susceptible to exogenous free radical abuses [39].
In CLL, malignant cells are in general more active in the production of ROS than normal cells and are under intrinsic OS. Thus, they are more vulnerable to damage by ROS-generating agents [19]. This is particularly true for late stage patients and those that have undergone through prior therapy, since mitochondrial defects seem to accumulate over time and after chemotherapy [40]. The intrinsic OS in cancer cells has been associated with the upregulation of SOD and CAT protein expression in primary CLL leukemia cells cultures [41]. However, studies published until today in CLL reveal discrepancies in information recompiled about OS in CLL patients in relation to changes of enzymatic activities and the products of peroxidation [12, 13, 21]. CLL cells at different disease stages may have different metabolic activities and thus produce various levels of superoxide radicals, depending on the energy requirement by the leukemia cells. Patients’ individual variations such as the intake of antioxidants and other medication might also contribute to the variation in free radical contents observed in CLL cells from different patients. To date, there has been no attempt to determine the OS status of early stage CLL and it is possible that these may present imbalance between the mechanisms that generate ROS and antioxidant defense mechanisms in favor of the latter, and thus show an increased oxidative distress in comparasion to the healthy population. The measurement of OS biomarkers in early stage CLL patients could be helpful in the search of accurate prognostic markers that allow early treatment for these patients.
The routine techniques applied in our laboratory are based on venous blood samples easily obtained and on not very sophisticated technical requirements. They permit to assess directly or indirectly the amount of ROS produced and also permit to determine the activity, the availability and effectiveness of protection of the antioxidant systems. In the present study, we analyzed different individual OS biomarkers with an antioxidant activity such as the low molecular weight antioxidants enzymes and macromolecules (SOD, CAT, GR, GPx, GSH) and also biomarkers of prooxidant status such as the presence of some products of protein and lipid peroxidation (GSSG, TBARS) [30]. As compared to healthy individuals, our results indicate that these biomarkers would be effective to confirm the existence of OS in early stage CLL patients prior treatment since we demonstrated that these patients present a significant increase of the prooxidant biomarkers (GSSG, ratio GSSG/GSH) and a significant decrease in the antioxidant biomarkers (SOD, CAT, GR, GPx). These results are in agreement with previous studies which present GSSG as a good biomarker of oxidative damage to peptides [42, 43]. The decrease of the antioxidant GSH and the increase of the products of peroxidation TBARS were not significant in our CLL patients. However, there is a significant decrease in erythrocyte TBARS level. The global antioxidant capacity determined by the ORAC method correlated positively with the prooxidant TBARS and GSSG, and negatively with the antioxidant enzymes SOD and GPx. This fact suggests a compensatory mechanism of the body in front of a radicalar attack [44-46]. Nevertheless, our results indicate that the assessment of a specific biomarker linked to OS remains insufficient to determine the state of prooxidant-antioxidant balance in normal or pathological situation, and it gives us very little information on where the imbalance is located. The fact that the assessement of individual biomarkers for the determination of OS status appear unwelcome has also been confirmed in previous studies [42].
The second step of the study consisted in the determination of a more realistic and comprehensive method by applying the method defined by Romeu and collaborators [47]. With this method of SOS we have been able to evaluate the balance status between the formation or ROS (prooxidant status) and the defensive systems (antioxidant status) in blood samples of early stage CLL patients and healthy control individuals. SOS values get a new variable that gives us the weight of the OS of the CLL patients without knowing more complex data for all biomarkers and their role in the cell. The SOS included all the OS biomarkers except plasmatic GSH, GSSG, GSSG/GSH due to conflicting results in the literature [37, 38]. The global antioxidant capacity determined with the ORAC has been also omitted since it is an overall assessment of OS as SOS. Our results highlighted a significant higher SOS in CLL patients as compared to healhty individuals. This may be considered solely attributable to the disease since none of the individuals had concomitant diseases and had not received any treatment that could produce a greater OS. However, not all patients in the population showed the same OS profile (Fig. 2). This is probably due to the high complexity of the global system and also to the intraindividual variability influenced by multiple genetic, metabolic and environmental factors [48, 49]. Although the SOS has been obtained in only one sampling point, its inclusion in the routine control analysis package would allow us to see the OS progression in these patients and also to determine with greater precision the progression of the disease through a longitudinal prospective study.
At this moment there is not enough evidence to initiate treatment in early stage CLL only on the basis on the presence of single classical and new adverse prognostic factors [50]. In our study, the analysis of the OS values in CLL patients according to whether they had favorable or adverse prognostic markers showed no statistical differences. However, interesting trends support the idea that the presence of a certain adverse prognostic factors (immunphenotypic score, CD38, ZAP-70, karyotype and β-2M) was associated with increased OS. Previous publications showed that the combination of various adverse prognostic factors in these patients led to a worse progression of the patient [4-6]. On the other hand, some prognostic index for patients with CLL have already been proposed, but this index does not provide any parameters related to OS [6, 7]. We used the NAPF as a variable that encompasses all the prognostic factors used in clinical practice today and we analysed the distribution of the value of the OS biomakers and SOS in function of this new variable. This combination reveals the existence of a group of patients with a favorable prognosis (NAPF of 0 and SOS of 1) and a group of patients with an adverse prognosis (NAPF of 3-4 and SOS of 5). Patients with intermediate SOS (2 or 3 points) would have a more uncertain prognosis.
Studies need sufficient patients to ensure statistical power. This fact can explain why many of our results did not present statistical significance. However, the number of patients included in our study within the planned recruitment period appears to be proportional to those observed in other studies with a longer period of time [5-7].
To incorporate the SOS as a prognostic factor in CLL, firstly, we should increase the number of patients included in the study and, secondly, we should follow patients over time. The latter approach would allow us to see what the real performance of individuals is and check if the "adverse/favorable prognosis" of each patient is in agreement with what has actually happened.
In conclusion, the SOS may be useful in patients with early stage CLL because it is a new variable of information about the degree of OS in the routine controls of CLL and it provides information on the prooxidant-antioxidant balance status at any time of the disease. According to the presence or not of prognostic factors, SOS can differentiate populations with different levels of OS. Finally SOS could provide information about possible chemotherapy treatment, considering that some of these uses the way of free radicals as a mechanism of action and it could open the door to a possible prescription of antioxidant therapy, either pharmacological or dietetical in patients with early stage CLL who presents adverse prognostic markers.
Acknowledgments
No.
Authorship and Disclosures
XO, MG and MR were the principal investigators and take primary responsibility for the paper. MRL, TS and LLF recruited the patients. MRN and VSM performed the laboratory work for this study. XO and ML wrote the paper. The authors reported no conflicts of interest.
Declaration
No dedication and funding.
| References | ▴Top |
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219-234.
pubmed - Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198-206.
doi - Parker TL, Strout MP. Chronic lymphocytic leukemia: prognostic factors and impact on treatment. Discov Med. 2011;11(57):115-123.
pubmed - Shanafelt TD, Geyer SM, Kay NE. Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103(4):1202-1210.
doi pubmed - Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S, Else M, et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95(10):1705-1712.
doi pubmed - Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Cortes J, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109(11):4679-4685.
doi pubmed - Molica S, Mauro FR, Callea V, Giannarelli D, Lauria F, Rotoli B, Cortelezzi A, et al. The utility of a prognostic index for predicting time to first treatment in early chronic lymphocytic leukemia: the GIMEMA experience. Haematologica. 2010;95(3):464-469.
doi pubmed - Meuleman N, Stamatopoulos B, Dejeneffe M, El Housni H, Lagneaux L, Bron D. Doubling time of soluble CD23: a powerful prognostic factor for newly diagnosed and untreated stage A chronic lymphocytic leukemia patients. Leukemia. 2008;22(10):1882-1890.
doi pubmed - Durig J, Nuckel H, Cremer M, Fuhrer A, Halfmeyer K, Fandrey J, Moroy T, et al. ZAP-70 expression is a prognostic factor in chronic lymphocytic leukemia. Leukemia. 2003;17(12):2426-2434.
doi pubmed - Montserrat E. New prognostic markers in CLL. Hematology Am Soc Hematol Educ Program. 2006:279-284.
doi pubmed - Van Bockstaele F, Verhasselt B, Philippe J. Prognostic markers in chronic lymphocytic leukemia: a comprehensive review. Blood Rev. 2009;23(1):25-47.
doi pubmed - Letestu R, Levy V, Eclache V, Baran-Marszak F, Vaur D, Naguib D, Schischmanoff O, et al. Prognosis of Binet stage A chronic lymphocytic leukemia patients: the strength of routine parameters. Blood. 2010;116(22):4588-4590.
doi pubmed - Battisti V, Maders LD, Bagatini MD, Santos KF, Spanevello RM, Maldonado PA, Brule AO, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41(7-8):511-518.
doi pubmed - Cerutti P, Ghosh R, Oya Y, Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ Health Perspect. 1994;102 Suppl 10:123-129.
pubmed - Masutani H. Oxidative stress response and signaling in hematological malignancies and HIV infection. Int J Hematol. 2000;71(1):25-32.
pubmed - Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1-40.
doi pubmed - Romeu M, Mulero M, Giralt M, Folch J, Nogues MR, Torres A, Fortuno A, et al. Parameters related to oxygen free radicals in erythrocytes, plasma and epidermis of the hairless rat. Life Sci. 2002;71(15):1739-1749.
doi - Lightfoot TJ, Skibola CF, Smith AG, Forrest MS, Adamson PJ, Morgan GJ, Bracci PM, et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin's lymphoma. Haematologica. 2006;91(9):1222-1227.
pubmed - Hileman EO, Liu J, Albitar M, Keating MJ, Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol. 2004;53(3):209-219.
doi pubmed - Links M, Lewis C. Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs. 1999;57(3):293-308.
doi pubmed - Devi GS, Prasad MH, Saraswathi I, Raghu D, Rao DN, Reddy PP. Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin Chim Acta. 2000;293(1-2):53-62.
doi - Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456.
doi pubmed - Moreau EJ, Matutes E, A'Hern RP, Morilla AM, Morilla RM, Owusu-Ankomah KA, Seon BK, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997;108(4):378-382.
pubmed - Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170-3175.
pubmed - Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30-38.
- Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW, Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem. 1990;184(2):193-199.
doi - Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214-226.
doi - El-Rashidy FH, Al-Turk WA, Stohs SJ. Glutathione, glutathione reductase and glutathione S-transferase activities in erythrocytes and lymphocytes in chronic renal disease. Res Commun Chem Pathol Pharmacol. 1984;44(3):423-430.
pubmed - Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489-492.
pubmed - Ortin F, Giralt M, Cervello I, Nogues MR, Puerto AM, Argany N, Mallol J. [The kinetic characteristics of the erythrocyte glutathione S-transferase system as a function of sex and the tobacco habit]. Med Clin (Barc). 1996;106(16):607-610.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310.
- Richard MJ, Portal B, Meo J, Coudray C, Hadjian A, Favier A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin Chem. 1992;38(5):704-709.
pubmed - Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68(5):1081-1087.
pubmed - Romeu M, Nogues R, Marcas L, Sanchez-Martos V, Mulero M, Martinez-Vea A, Mallol J, et al. Evaluation of oxidative stress biomarkers in patients with chronic renal failure: a case control study. BMC Res Notes. 2010;3:20.
- Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007;380(1-2):50-58.
doi pubmed - Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187-197.
doi pubmed - Hampton MB, Fadeel B, Orrenius S. Redox regulation of the caspases during apoptosis. Ann N Y Acad Sci. 1998;854:328-335.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. Oxford, United Kingdom: Oxford University Press; 1999.
- Hileman EA, Achanta G, Huang P. Superoxide dismutase: an emerging target for cancer therapeutics. Expert Opin Ther Targets. 2001;5(6):697-710.
doi pubmed - Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101(10):4098-4104.
doi pubmed - Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65(2):613-621.
pubmed - Dotan Y, Lichtenberg D, Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res. 2004;43(3):200-227.
doi pubmed - Griffiths HR, Moller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, Cooke M, et al. Biomarkers. Mol Aspects Med. 2002;23(1-3):101-208.
doi - Lodovici M, Bigagli E, Bardini G, Rotella CM. Lipoperoxidation and antioxidant capacity in patients with poorly controlled type 2 diabetes. Toxicol Ind Health. 2009;25(4-5):337-341.
doi pubmed - Lodovici M, Giovannelli L, Pitozzi V, Bigagli E, Bardini G, Rotella CM. Oxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic control. Mutat Res. 2008;638(1-2):98-102.
doi pubmed - Lin TK, Chen SD, Wang PW, Wei YH, Lee CF, Chen TL, Chuang YC, et al. Increased oxidative damage with altered antioxidative status in type 2 diabetic patients harboring the 16189 T to C variant of mitochondrial DNA. Ann N Y Acad Sci. 2005;1042:64-69.
- Romeu M. Distrès oxidatiu en humans. Valoració en diferents situacions fisiològiques i patològiques. [Doctoral Thesis]. Reus: Universitat Rovira I Virgili; 2006.
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46(5-6):241-281.
doi pubmed - Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321-329.
doi pubmed - Montserrat E, Moreno C, Esteve J, Urbano-Ispizua A, Gine E, Bosch F. How I treat refractory CLL. Blood. 2006;107(4):1276-1283.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


