| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 1, January 2018, pages 19-22
A Challenging Blastic Plasmacytoid Dendritic Cell Neoplasm Case: Tough Decisions to Make
Joseph Hana, Faisal Huq-Ronnyb, Maher Abdul-Haya, c, d
aDepartment of Medicine, New York University School of Medicine, New York, NY, USA
bDepartment of Hematopathology, New York University School of Medicine, New York, NY, USA
cNew York University Perlmutter Cancer Center, New York, NY, USA
dCorresponding Author: Maher Abdul-Hay, New York University School of Medicine, 19 Floor, 240 East 38th Street, New York, NY 10016, USA
Manuscript submitted November 27, 2017, accepted December 15, 2017
Short title: BPDCN
doi: https://doi.org/10.14740/jh355w
| Abstract | ▴Top |
Blastic plasmacytoid dendritic cell neoplasm is a very rare disease and in our case report we discuss the presentation of our patient, her treatment and response in addition for a discussion of the newly therapeutic options that are available.
Keywords: Blastic plasmacytoid dendritic cell neoplasm; Leukemia; Hyper CVAD-methotrexate-cytarabine chemotherapy
| Introduction | ▴Top |
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a very aggressive type of cancer with multiple manifestations including skin lesions, abnormal cell counts and central nervous system (CNS) disease involvement. In our case report, we discuss a patient with BPDCN, her treatment and prognosis; in addition, we highlight the available treatments and the future novel agents coming up for the treatment of BPDCN.
| Case Report | ▴Top |
Our patient is a 23-year-old Chinese female with no significant past medical history that presented in September 2016 with worsening facial rash that started about 2 months before presentation. The patient first noted a “red mark” on her cheek, which improved initially but then recurred shortly after. She saw a dermatologist at an outside hospital where a biopsy of the rash was consistent with myeloid sarcoma. However, her treatment was delayed because she initially had no access to healthcare. Once the patient received insurance, her primary care provider recommended presentation for admission for further workup. When she was admitted for workup she was noted to be pregnant in her early first trimester.
Her examination revealed diffuse erythematous and purpuric lesions overlying her face predominantly (cheeks bilaterally, eyelids, and forehead) with additional erythematous lesions scattered across her chest, back, abdomen, and legs (Fig. 1). No scaling, skin bleeding, or purulent drainage was noted. Denied any pain or pruritus associated with the rash. No palpable lymphadenopathy or hepatosplenomegaly. Laboratory results revealed a hemoglobin level of 90 g/L, white blood cell count of 2.5 × 109/L and platelet count of 1.81 × 1010/L. Blood chemistry and coagulation tests were unremarkable. LDH level was elevated at 351.
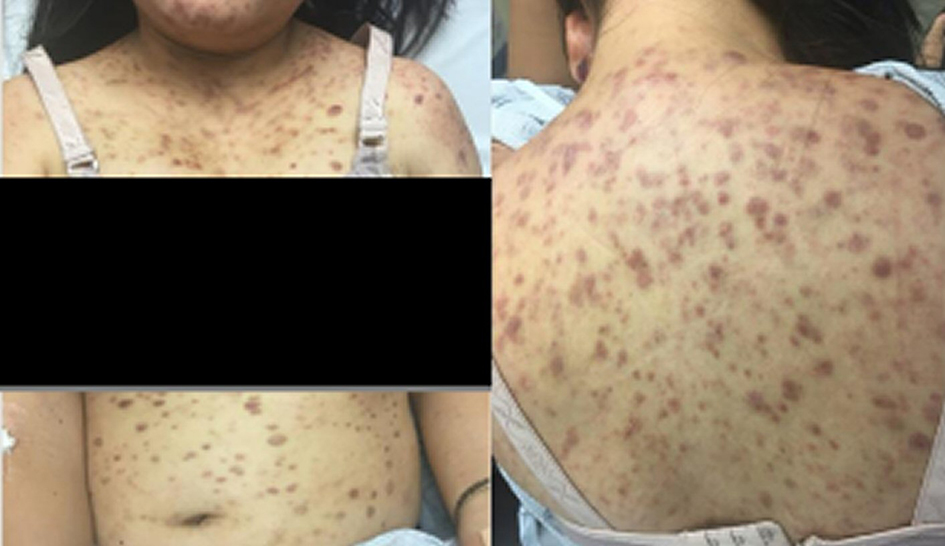 Click for large image | Figure 1. Diffuse macular and erythematous lesions scattered on chest and abdomen (left), and back (right). |
Biopsy results revealed dense infiltrate of atypical medium to large cells. By immunohistochemistry, the neoplastic cells have mild immunoreactivity to CD45, were positive for CD4, CD56, and CD43. No B cells as assessed by CD20 and very few scattered CD3+ T cells. CD30 is negative, Ki-67 proliferative index estimated > 95%. CD2, CD7, CD5, CD8, S100, CD34, CD117, MPO, CD68, EBV, (LMP1), MUM1, synaptophysin, and chromogranin were negative.
Patient underwent bone marrow (BM) aspiration (Fig. 2) and biopsy (Fig. 3). PB and BM smears were performed, and the results demonstrated that blastic or abnormal cells accounted for 53% of the nucleated cells. As seen in Figure 2, the abnormal BM cells were found to exhibit eccentric, large, round-to-oval/convoluted nuclei with mostly fine chromatin and prominent nucleoli and moderate-to-abundant gray cytoplasm with several cells exhibiting multiple diminutive cytoplasmic vacuoles. BM biopsy demonstrated a markedly hypercellular marrow with multiple interstitial aggregates of spindled mononuclear cells with dark smudged chromatin moderate to abundant gray cytoplasm, which comprise approximately 60-70% of the total cellularity of the specimen. Based on the results of immunohistochemical analysis, the cells are TdT(+), CD56(+), CD123(+) and negative for myeloperoxidase (MPO), CD34, CD117, CD20, CD3 and EBV. Flow cytometry (Fig. 4) revealed 57% medium to large abnormal CD45(dim+) cells within the nuclear cell gate with atypical cells positive for CD45 (slightly dim+), CD4 (dim+), CD13 (-), CD15(-), CD33(+), CD34(-), CD36 (partial+), CD38 (partial+), CD117 (variably+), HLADR(+), CD14(-), CD64(-), CD56(+), and CD123(+) which was compatible with BPDCN. Diagnosis of BPDCN was confirmed by the positivity of the cells for CD4 and CD56, along with other markers that are more restricted to plasmacytoid dendritic cells (such as CD123), and the negativity of the cells for lymphoid, NK and myeloid lineage-associated antigens.
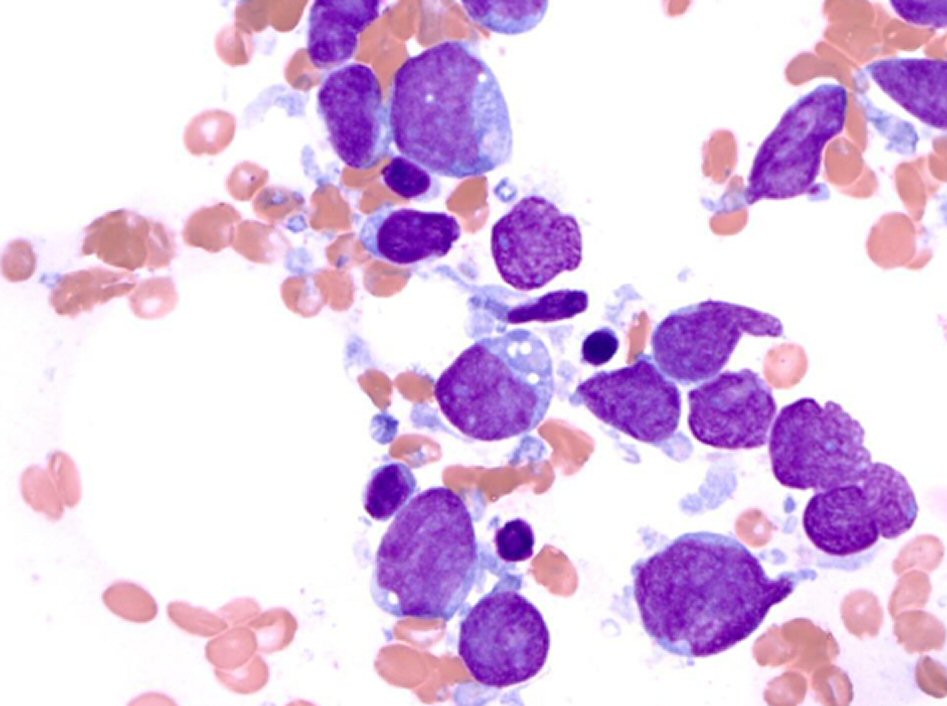 Click for large image | Figure 2. Bone marrow aspiration results: Wright-Giemsa staining (magnification, × 1,000). |
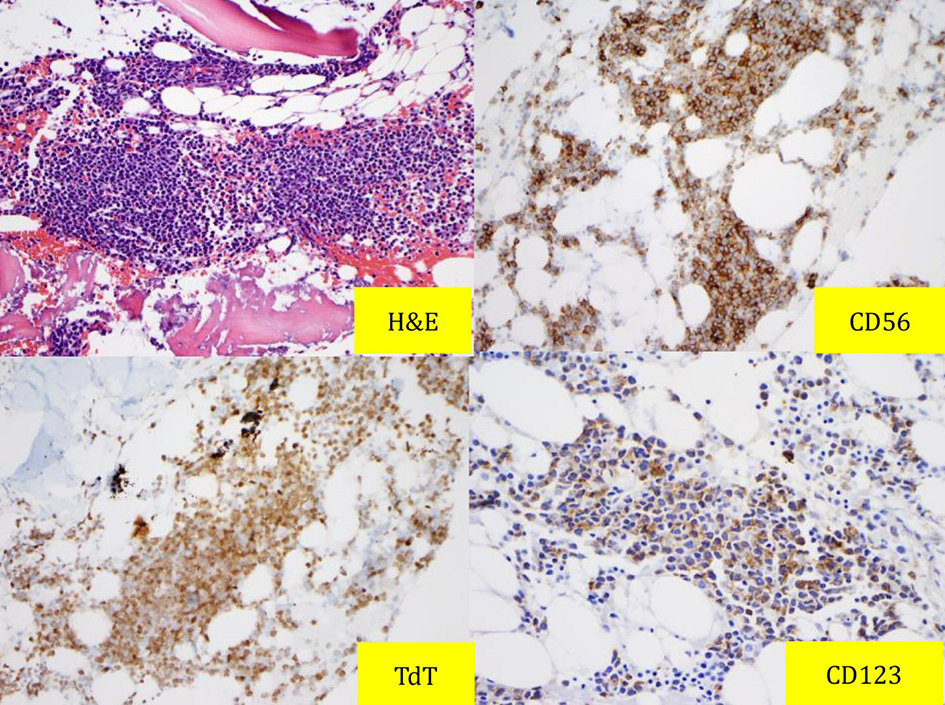 Click for large image | Figure 3. Bone marrow biopsy results: H&E and immunostaining with CD56, TdT and CD123. |
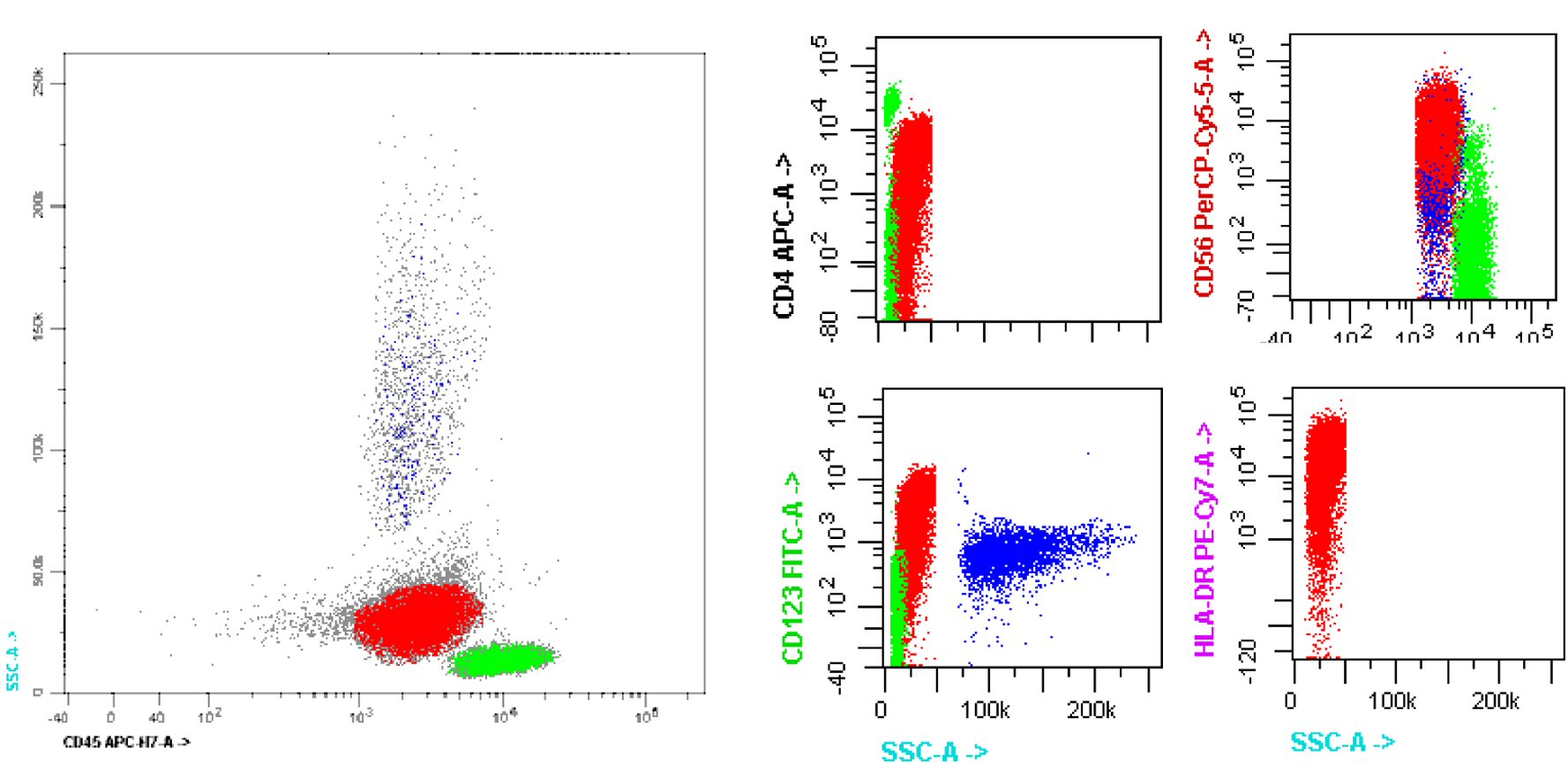 Click for large image | Figure 4. Flow cytometric immunophenotyping. Side scatter (SS) by CD45 flow cytometric profile of the blastic plasmacytoid dendritic cell neoplasm blast gate (red), demonstrating expression of CD4, CD56, CD123 and HLA-DR. HLA-DR: human leukocyte antigen-DR. |
Given the aggressive nature of BPDCN and the need for cytotoxic chemotherapy the patient elected to terminate her pregnancy which was a difficult emotional decision for her and her spouse to make. The patient was treated on an acute lymphoblastic leukemia (ALL) protocol for eight cycles of hyperCVAD (cyclophosphamide, vincristine, adriamycin and decadron) alternating with MTX/Ara-C (methotrexate and cytarabine) and achieved complete remission (CR) confirmed by a bone marrow biopsy and by the resolution of most of her skin lesions.
Her first cycle of treatment was complicated by persistent epistaxis and neutropenic fever. She tolerated the remaining cycles of treatment well with another admission for neutropenic fever at her last cycle of treatment. Given high risk of relapse patient was evaluated during her treatment for allogeneic stem cell transplant and in the process of having it done.
| Discussion | ▴Top |
In the present case, the diagnosis of BPDCN was challenging due to the absence of traditional lineage-specific markers and typical cutaneous presentation. However, the results of flow cytometric analysis identified the typical CD45dim+, CD4dim+, CD56+, HLA-DR+, and CD123+ immunophenotype. These observations, along with the characteristic morphology and immunologic findings in the present case, indicated that the disease was consistent with the features of leukemic BPDCN.
At present, there is no consensus regarding an optimal standardized therapeutic approach for BPDCN. This is due to the low incidence of this disease, consequently resulting in a lack of prospective data and randomized controlled clinical trials. Regardless of the type of therapy, the prognosis has been a dismal reported median overall survival of 12 - 16 months and high rate of relapse within 1 year [1-3]. Patients with early-stage disease limited to the skin have a better prognosis, and these patients may have a possible survival benefit from radiotherapy alone [4]. However, the majority of patients present with advanced disease, requiring early and aggressive therapy.
Published studies suggest that patients who receive leukemia-based regimens obtain better responses than patients who receive non-Hodgkin lymphoma based regimens [5, 6]. More specifically, studies have shown a relative superiority of ALL-type induction regimens over AML in terms of both response and duration of response [7]. Pagano et al had the largest retrospective study to date comparing the response of acute lymphoid leukemia/lymphoma-type regimen versus acute myeloid leukemia-type regimen in 41 cases of BPDCN. There was a significant difference in the percentage of patients who achieved a CR (0.67 vs. 0.27 respectively, P = 0.02) and a higher median overall survival (12.3 months versus 7.1 months, P = 0.02) with an ALL based protocol [8]. A more recent retrospective study compared ALL versus AML versus lymphoma-type therapy and found that ALL-type therapy had the lowest rates of relapse and overall mortality [9]. However, these results may be potentially confounded by the inclusion of children in the cohort.
Although BPDCN patients initially achieve a complete or partial response to chemotherapy, many succumb to a relapse within 2 years regardless of the type of chemotherapy they received. The relapsed disease is often resistant to the chemotherapy agents previously used [3]. There is also a higher risk of CNS involvement during disease recurrence after CR (33%) [1]. CNS prophylaxis with intrathecal drug infusions that can penetrate the CNS brain barrier should be included during induction treatment, as these patients have been observed to have both prolonged CNS recurrence free disease and overall survival rate [10] and our patient received multiple CNS prophylactic treatments.
One promising solution to increase long-term overall survival has been in the area of allogeneic hematopoietic stem cell transplantation (HCST). Retrospective case reports suggest that adults may benefit from consolidation with allogeneic HSCT during their first CR following chemotherapy with one study observing a median overall survival of 38.5 months [5]. The best outcome for HSCT is to perform it after the first CR as it had been noted in studies to have a significantly lower overall survival and progression-free survival when done after the first CR [5, 11]. In regards to the optimal conditioning regimen, myeloablative conditioning (MAC) was associated with a higher 3-year disease-free survival versus a reduced intensity conditioning (RIC) regimen [12]. However, it should be noted that the number of patients who received RIC was a smaller group and those patients were significantly older.
Until recently, there have been few published cases on the role of autologous HSCT. Aoki et al conducted a retrospective analysis on a cohort of 25 BPDCN patients showing that the group who received autologous versus allogeneic HSCT, following high dose chemotherapy, tended to have a higher 4-year overall survival rate (0.82 vs. 0.53 respectively, P = 0.11) and higher progression-free survival rate (0.73 vs. 0.48 respectively, P = 0.14) [11]. Although not statistically significant, the results are intriguing to warrant further investigation with a larger study.
While the combination of high dose chemotherapy followed by HSCT during first CR has been favorable for a longer overall survival, it is an intense therapy that may not be suitable to all patients (i.e. elderly). It is necessary to have other alternative therapeutic approaches. One drug that appears promising is SL-401, a recombinant protein consisting of a fusion between a truncated diphtheria alpha-toxin and human interleukin 3-alpha (IL3A). BPCDN cells have a high expression of the IL3 receptor (CD123), allowing selective receptor-mediated binding and endocytosis of the cytotoxin. Its mechanism is not cell-cycle dependent, allowing it to selectively target both proliferating and dormant malignant cells while sparing normal marrow progenitors [13].
SL-401 has been tested in a phase 1/2 study of 11 patients with BPDCN. Each patient received one course, which consisted of 5 days of 15-minute infusions of SL-401 at 12.5 µg/kg. Seven out of the nine evaluable patients had objective responses of five with CR and two with partial remission (PR). Median duration of response was 5 months. Adverse events were minimal and brief; with no treatment related deaths [14]. Another trial evaluated the use of SL-401 in nine patients with relapsed/refractory BPDCN. Four out of the seven evaluable patients achieved CR [15]. These promising results have led to a larger ongoing prospective study of SL-401 (NCT02113982).
Conclusions
Our patient was a challenging case given time to diagnose her and given her pregnancy, however she ended up doing well and having a great response for treatment based on an ALL protocol. BPDCN is a very rare disease < 1% of all acute leukemias and accordingly carriers have the burden for diagnosis and treatment. We are hoping that our patient after her allogeneic stem cell transplant soon enough and she will be able to expand her family members and enjoy a prosperous life with them.
Disclosures
None.
| References | ▴Top |
- Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, Arnoulet C, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556-1563.
doi pubmed - Bekkenk MW, Jansen PM, Meijer CJ, Willemze R. CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004;15(7):1097-1108.
doi pubmed - Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138(4):564-569.
doi pubmed - Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. G Ital Dermatol Venereol. 2012;147(6):603-608.
pubmed - Reimer P, Rudiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, Konrad Muller-Hermelink H, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32(7):637-646.
doi pubmed - Kharfan-Dabaja MA, Lazarus HM, Nishihori T, Mahfouz RA, Hamadani M. Diagnostic and therapeutic advances in blastic plasmacytoid dendritic cell neoplasm: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1006-1012.
doi pubmed - Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, Pappa V, et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34(4):438-446.
doi pubmed - Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, Lunghi M, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239-246.
doi pubmed - Martin-Martin L, Lopez A, Vidriales B, Caballero MD, Rodrigues AS, Ferreira SI, Lima M, et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget. 2015;6(22):19204-19216.
doi pubmed - Martin-Martin L, Almeida J, Pomares H, Gonzalez-Barca E, Bravo P, Gimenez T, Heras C, et al. Blastic plasmacytoid dendritic cell neoplasm frequently shows occult central nervous system involvement at diagnosis and benefits from intrathecal therapy. Oncotarget. 2016;7(9):10174-10181.
doi pubmed - Aoki T, Suzuki R, Kuwatsuka Y, Kako S, Fujimoto K, Taguchi J, Kondo T, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125(23):3559-3562.
doi pubmed - Roos-Weil D, Dietrich S, Boumendil A, Polge E, Bron D, Carreras E, Iriondo Atienza A, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121(3):440-446.
doi pubmed - Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62(6):1730-1736.
pubmed - Frankel AE, Woo JH, Ahn C, Pemmaraju N, Medeiros BC, Carraway HE, Frankfurt O, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385-392.
doi pubmed - Sweet KL, Pemmaraju N, Lane AA, et al. Lead-in stage results of a pivotal trial of SL-401, an Interleukin-3 Receptor (IL-3R) targeting biologic, in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) or Acute Myeloid Leukemia (AML). Blood. 2015;126(23):3795.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


