| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 1, January 2018, pages 29-31
Clinical Resolution of Red Cell Aplasia Associated del 17p Multiple Myeloma, When Treated With Bortezomib and Dexamethasone
Abdullah Ladhaa, d, Yelena Fudymb, Teresa C. Gentilec
aDepartment of Hematology and Oncology, State University of New York (SUNY) Upstate Medical University, 750 E Adam St, Syracuse, NY 13210, USA
bDepartment of Pathology, State University of New York (SUNY) Upstate Medical University, 750 E Adam St, Syracuse, NY 13210, USA
cDepartment of Hematology and Oncology, State University of New York (SUNY) Upstate Medical University, 750 E Adam St, Syracuse, NY 13210, USA
dCorresponding Author: Abdullah Ladha, 750 E Adam St, Syracuse, NY, 13210, USA
Manuscript submitted November 5, 2017, accepted December 1, 2017
Short title: Red Cell Aplasia and del 17p Multiple Myeloma
doi: https://doi.org/10.14740/jh356w
| Abstract | ▴Top |
Red cell aplasia has been rarely described in association with multiple myeloma. We present a case of a 79-year-old female, who was initially diagnosed with iron deficiency anemia, which did not improve with iron supplementation and required blood transfusions. Bone marrow biopsy showed red cell aplasia associated with kappa light chain multiple myeloma with 14.8% plasma cells. Further tests showed 0.35 g/dL M protein, and kappa/lambda ratio was 131.84. Cytogenetic showed deletion 13q, deletion 17p, loss of 1p and gain of chromosome 5. Multiple myeloma directed treatment with bortezomib and dexamethasone was initiated. Patient had clinical resolution of anemia and did not require further blood transfusions. This is an intriguing case of red cell aplasia associated with poor risk multiple myeloma (del 17p), which showed clinical improvement in anemia with bortezomib-based therapy. This case highlights the role of clonal plasma cells proliferation in the pathogenesis of red cell aplasia as myeloma directed treatment helped patient to become transfusion independent.
Keywords: Pure red cell aplasia; Multiple myeloma; Plasma cell leukemia; Blood transfusion
| Introduction | ▴Top |
Red cell aplasia is characterized by reduction in erythroid precursors. It can be idiopathic, due to an autoimmune process or secondary to thymoma, lymphoid malignancy, viral infection or medications [1, 2]. There are rare case reports of red cell aplasia associated with plasma cell dyscrasia. Reports have described pure red cell aplasia (PRCA) in patients with monoclonal gammopathy of unknown significance (MGUS), IgG lambda and IgG kappa multiple myeloma, biclonal multiple myeloma with IgG lambda and IgA lambda, light chain disease and IgM kappa and IgM lambda Waldenstrom macroglobulinemia [3-11]. The effect of myeloma treatment on red cell aplasia is found to be inconsistent in literature; some studies suggested improvement in red cell aplasia with myeloma treatment and some showed response to immunosuppression [3-5]. It has been suggested that plasma cell clones generate antibodies to interrupt erythropoiesis and also an autoimmune mechanism has also been suggested [3-5, 12]. Interaction between plasma cell dyscrasia and red cell aplasia is not well understood. This association has also been described in patients with plasma cell dyscrasia (MGUS and smoldering myeloma) with normal karyotype and with inv 2p [12]. We describe a case of deletion 17p (high risk) multiple myeloma patient with associated red cell aplasia, which showed clinical resolution of red cell aplasia and did not require blood transfusion after treatment with bortezomib and dexamethasone.
| Case Report | ▴Top |
Patient was a 79-year-old Caucasian female, with history of hypertension, obstructive sleep apnea, and chronic obstructive pulmonary disease with Eastern Cooperative Oncology Group performance status of 1. She presented with iron deficiency anemia, with ferritin 6 ng/mL, iron saturation 6.2% and iron 28 mg/dL. Treatment with oral ferrous gluconate 324 mg three times a day was started. She continued to be anemic and required intravenous iron sucrose. At one point patient’s hemoglobin dropped less than 7 g/dL, requiring packed red blood cell transfusion. She had no rashes, arthralgias or juandice. Esophagogastroduodenoscopy and colonoscopy were unremarkable. CT scans were unremarkable. As anemia did not improve, a bone marrow biopsy was planned. It showed hyper-cellularity, numerous scattered plasma cells and absence of erythroid precursors. Bone marrow aspirate showed 14.8% clonal plasma cells (Fig. 1). Cytogenetics revealed deletion 13q, deletion 17p, and loss of 1p and gain of chromosome 5. Serum protein electrophoresis showed discrete band of 0.35 g/dL in beta region, urine immunofixation showed monoclonal kappa light chain, patient had normal creatinine and calcium but her kappa/lambda ratio was 131.84. Skeletal survey was unremarkable. Patient was diagnosed with PRCA associated with kappa light chain multiple myeloma. She was classified high-risk and stage III based on revised international staging system. Treatment for multiple myeloma was started with bortezomib 1.3 mg/m2 subcutaneously on days 1, 4, 8 and 11 with dexamethasone 20 mg orally on days 1 - 2, 4 - 5, 8 - 9 and 11 - 12. Patient completed six cycles, showed improvement in anemia and did not require further packed red blood cell transfusion. Patient has been in remission since last 8 months.
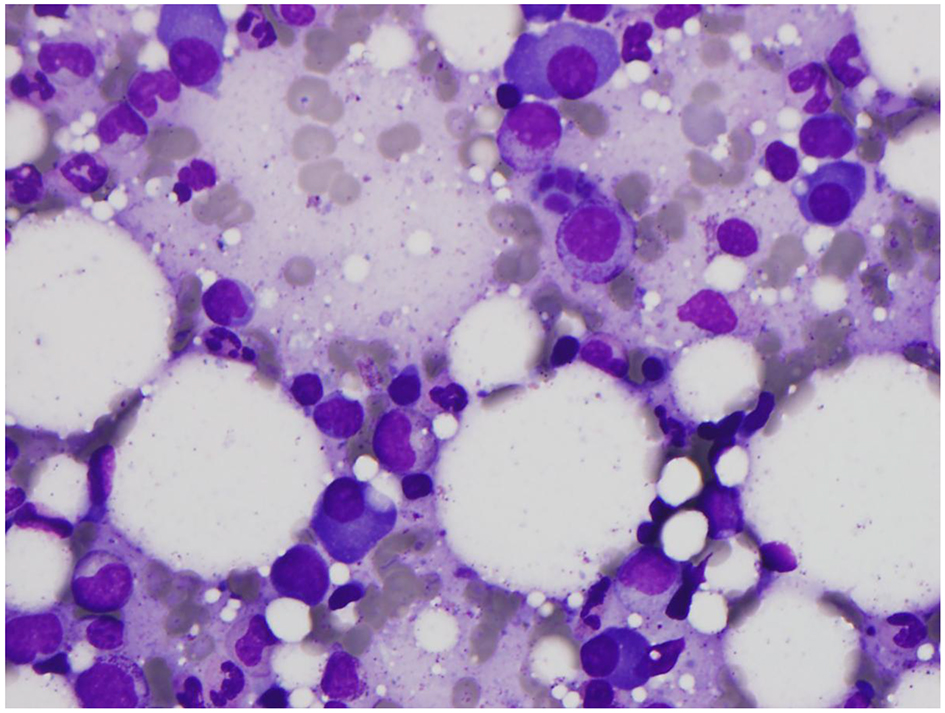 Click for large image | Figure 1. Bone marrow slide. Bone marrow aspirate showing increased mature plasma cells (14.8% marrow differential) with low N/C ratio, clumped chromatin, eccentric nuclei, inconspicuous nucleoli, and prominent perinuclear huff. Normal maturation of myeloid precursors is seen throughout with appropriate folding and granulation. Marked decrease of erythroid precursors is noted with complete absence in this field (M/E ratio 15.9/1.0). |
| Discussion | ▴Top |
PRCA has rarely been associated with plasma cell dyscrasia. Investigators at the National Institutes of Health (NIH) reviewed bone marrow biopsies of 51 patients with PRCA and found 12 patients with associated plasma cell dyscrasia, including SM and MGUS. They observed reversal of PRCA with plasma cell dyscrasia treatment in three patients, suggesting a functional relationship between red cell precursors and clonal plasma cells. The authors postulated that red cell aplasia might be related to clonal proliferation of plasma cells [12].
The interaction between PRCA and plasma cell dyscrasia is not well understood. Variable results have been reported on the effect of myeloma treatment on PRCA. So et al described CD20+ IgG lambda myeloma with red cell aplasia, which was treated with rituximab. It showed complete morphological response and remission of PRCA, but lower level of paraprotein persisted. They postulated that red cell aplasia might be caused by a subset of paraprotein or by a separate mechanism in addition to paraprotein mediated erythropoiesis suppression [6]. Similarly, Sarathy et al observed a decrease in blood transfusion and improvement of anemia when a patient with multiple myeloma was treated with bortezomib therapy [4]. Although Sarathy et al described reversal of red cell aplasia with bortezomib therapy, cytogenetics abnormalities were not discussed. In previously described NIH study, 11 out of 12 patient with plasma cell dyscrasia (MGUS and SM) and red cell aplasia had normal karyotype and one patient had inv 2p [12]. In the same study, three patients showed improvement in red cell aplasia with myeloma treatment. In our case, patient had high-risk changes including deletion of 13q, and 17p, loss of 1p and gain of chromosome 5 but had clinical improvement in anemia with proteasome inhibitor therapy [13].
Interestingly, Lv et al described a case of biclonal multiple myeloma with red cell aplasia and did not find improvement of anemia with myeloma treatment including bortezomib; however, the patient responded to immunosuppressive therapy [5]. Similarly, Krantz and Kao also described a case with PRCA and IgG lambda gammopathy of unknown significance, patient’s PRCA responded to immunosuppression without treatment of the MGUS. Therefore, it was concluded that MGUS did not play any role in PRCA [3]. In both of these reports, patients’ cytogenetic abnormalities were not discussed.
Clinical resolution of red cell aplasia with bortezomib and dexamethasone in poor risk del 17p multiple myeloma patient is very intriguing and has not been described previously. This case report suggests that the clonal plasma cell proliferation can interfere with normal erythropoiesis and lead to PRCA. Although corticosteroids are part of PRCA treatment but steroids alone are not adequate and result in 80% relapse rate on tapering doses [2]. Treatment of multiple myeloma does not require continuous daily doses and also does not require tapering. As patient has been in remission since last 8 months after completing therapy and has not required blood transfusions, it indicates that patient had improvement with myeloma directed treatment.
Conflict of Interest
Authors have no financial or other conflict of interest to disclose.
| References | ▴Top |
- Means RT Jr. Pure red cell aplasia. Blood. 2016;128(21):2504-2509.
doi pubmed - Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142(4):505-514.
doi pubmed - Krantz SB, Kao V. Studies on red cell aplasia. II. Report of a second patient with an antibody to erythroblast nuclei and a remission after immunosuppressive therapy. Blood. 1969;34(1):1-13.
pubmed - Sarathy KS, Ramakrishna R, Baig WW, Manoharan A. Acquired pure red cell aplasia in patients with plasma cell neoplasm and long term remission with Bortezomib therapy. J Hematol Malig. 2013;3(2):37-44.
- Lv Y, Qian W. Treatment of pure red cell aplasia associated with multiple myeloma with biclonal gammopathy using cyclosporine A: a case report. Int J Clin Exp Med. 2015;8(1):1498-1500.
pubmed - So CC, Choi WW, Kwong YL. Pure red cell aplasia associated with CD20+ myeloma: complete remission with rituximab. Ann Hematol. 2013;92(10):1425-1426.
doi pubmed - Li YY, Fan L, Wang L, Xu J, Li JY, Xu W. Waldenstrom's macroglobulinaemia complicated by pure red cell aplasia: a case report. Blood Transfus. 2013;11(4):630-633.
pubmed - Orchard J, Myint H, Hamblin TJ. A patient with myeloma who still has pure red cell aplasia despite the most intensive immune modulation. Leuk Res. 1997;21(4):353-354.
doi - Karmochkine M, Oksenhendler E, Leruez-Ville M, Jaccard A, Morinet F, Herson S. Persistent parvovirus B19 infection and pure red cell aplasia in Waldenstrom's macroglobulinemia: successful treatment with high-dose intravenous immunoglobulin. Am J Hematol. 1995;50(3):227-228.
doi pubmed - Resegotti L, Dolci C, Palestro G, Peschle C. Paraproteinemic variety of pure red cell aplasia: immunological studies in 1 patient. Acta Haematol. 1978;60(4):227-232.
doi pubmed - Gilbert EF, Harley JB, Anido V, Mengoli HF, Hughes JT. Thymoma, plasma cell myeloma, red cell aplasia and malabsorption syndrome. Am J Med. 1968;44(5):820-829.
doi - Korde N, Zhang Y, Loeliger K, Poon A, Simakova O, Zingone A, Costello R, et al. Monoclonal gammopathy-associated pure red cell aplasia. Br J Haematol. 2016;173(6):876-883.
doi pubmed - Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7):719-734.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


