| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 1, January 2018, pages 38-42
Mantle Cell Lymphoma Relapsing as Disease of Skin, Orbit and CNS: An Extremely Rare Presentation and a Review of Literature
Hira Shaikha, Prashant Janib, d, Rupin Shahb, Farshaad Bilimoriac, Jeffry Uchinc, Prerna Mewawallab
aDepartment of Medicine, Allegheny Health Network, Pittsburgh, PA, USA
bDivision of Hematology-Oncology, Allegheny Health Network, Pittsburgh, PA, USA
cDepartment of Pathology, Allegheny Health Network, Pittsburgh, PA, USA
dCorresponding Author: Prashant Jani, Allegheny General Hospital, Allegheny Cancer Center, 320 E North Ave., Pittsburgh, PA 15212, USA
Manuscript submitted December 3, 2017, accepted December 15, 2017
Short title: Relapse MCL of Skin, Orbit and CNS
doi: https://doi.org/10.14740/jh363w
| Abstract | ▴Top |
Mantle cell lymphoma (MCL) is a form of non-Hodgkin’s lymphoma originating from mature B cells. The hallmark gene translocation (11:14) results in overexpression of cyclin D1. Affected extranodal sites include bone marrow and gastrointestinal tract, but skin, orbit or CNS are rarely involved. Twenty-four cases have reported involvement of skin by MCL, while orbital MCL is equally rare. Our case is the first to report relapsed MCL with involvement of the skin and orbit simultaneously without disease in the lymphatic system or the bone marrow. A 53-year-old female with stage IV MCL initially presented with pancytopenia, adenopathy and splenomegaly. She achieved complete remission after six cycles of rituximab and bendamustine. Within 4 weeks of treatment, she developed diplopia and a rash of the left breast. Skin biopsy showed lymphoma infiltrates with B-cell markers for MCL. MRI of the orbits and brain suggested orbital lymphoma. CSF cytology further confirmed MCL cells. At time of relapse, she continued to be in hematologic remission. She initiated intrathecal cytarabine and methotrexate along with ibrutinib. R-CHOP was then added to the regimen. Within 2 weeks of starting treatment, her skin disease resolved and she had improvement in vision. MCL commonly presents as a disseminated disease, resulting in high mortality. Involvement of the skin or orbit has been sparingly reported and always suggests aggressive disease. It thus poses a challenge to diagnose and treat the condition as evidenced by resolution of adenopathy and bone marrow disease. Due to the overall poor prognosis of MCL and its unique presentations, as demonstrated by our case, early detection and prompt treatment are crucial to survival.
Keywords: Mantle cell; Relapse; Orbit; Skin; CNS
| Introduction | ▴Top |
Mantle cell lymphoma (MCL) comprises around 3-10% of non-Hodgkin lymphoma sub-types. It originates from the proliferation of mature B cells in the mantle zone of lymphoid follicles. Hallmarks of diagnosis that have been historically well known are translocation (11:14) and cyclin D1, the former causing overexpression of the latter [1, 2]. Over the time, CD5 and recently SOX11 have also become an essential part of the diagnosis [3, 4]. While classically known to be a nodal disease, it frequently affects extranodal sites such as bone marrow, gastrointestinal tract and Waldeyer’s ring, but skin, orbit and CNS are rarely involved.
MCL predominantly occurs in older males, frequently presenting at a widespread stage and has an aggressive clinical course. According to a study, around 70% have bone marrow invasion and 80% of patients are at stage III or IV with enlarged lymph nodes at the time of diagnosis [5]. Although it typically involves lymph nodes, involvement of extranodal organs is not uncommon. Systemic MCL generally has a poor median survival of 3 - 6 years after diagnosis [6].
| Case Report | ▴Top |
A 53-year-old female initially presented with pancytopenia, adenopathy and splenomegaly and was diagnosed with stage IV MCL. She was in complete remission after six cycles of rituximab and bendamustine. Within 4 weeks of the last cycle, she presented with blurry vision, diplopia and an erythematous nodular rash of the left breast. Skin biopsy showed lymphoma infiltrates positive for B-cell markers (CD20, CD79a, and PAX5), CD3, CD5, CD10, BCL2, BCL6 and cyclin D1, and exhibiting a Ki67 proliferation index approaching 100% (Figs. 1 and 2). MRI of the orbits exhibited bilateral optic nerve sheath involvement, while brain MRI showed enhancement within intraconal aspect of right orbit, suggestive of orbital lymphoma. CSF cytology further confirmed MCL, cells positive for CD19, CD20, CD22, CD5 and cyclin D1 on immunohistochemistry (Fig. 3). Flow cytometry revealed 93% of cells comprised of B cells with kappa light chain restriction and immunophenotype positive for CD5, CD10, CD19, CD20 and CD22, suggestive of CD5+ clonal lymphoproliferative disorder, consistent with diagnosis of MCL.
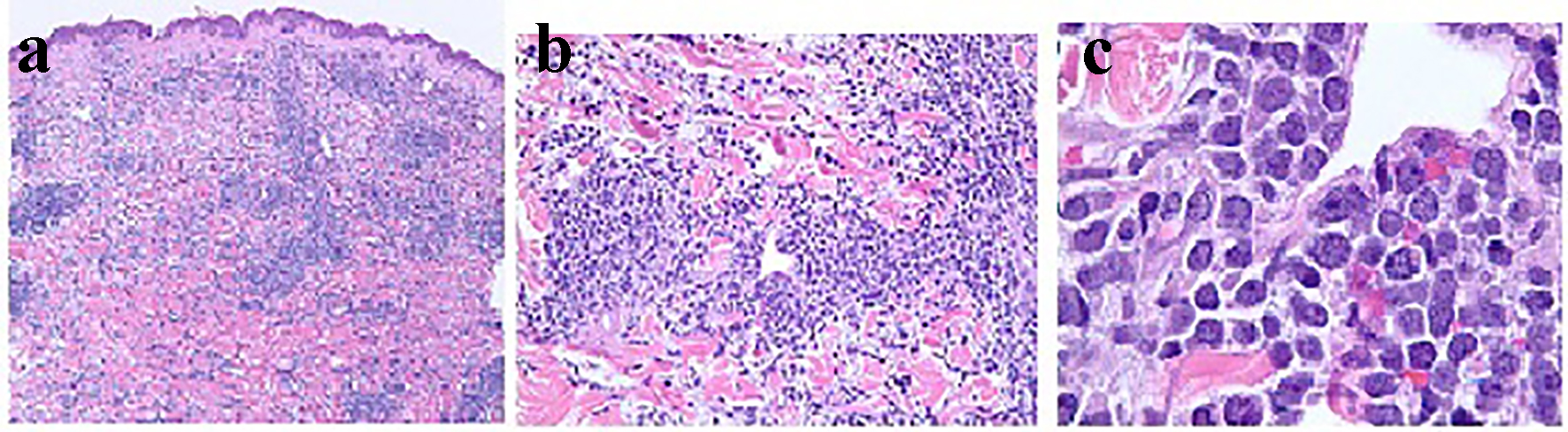 Click for large image | Figure 1. Histopathology of the skin on biopsy with × 4, × 20 and × 100 magnifications in (a), (b) and (c), respectively, demonstrating dense infiltrate of atypical lymphoid cells distributed throughout the dermis and subcutis. The atypical lymphocytes appear as small to medium sized, contain scant cytoplasm, and have hyperchromatic nuclei with irregular nuclear contours and occasional indistinct nucleoli. There are scattered apoptotic bodies and occasional mitotic figures. |
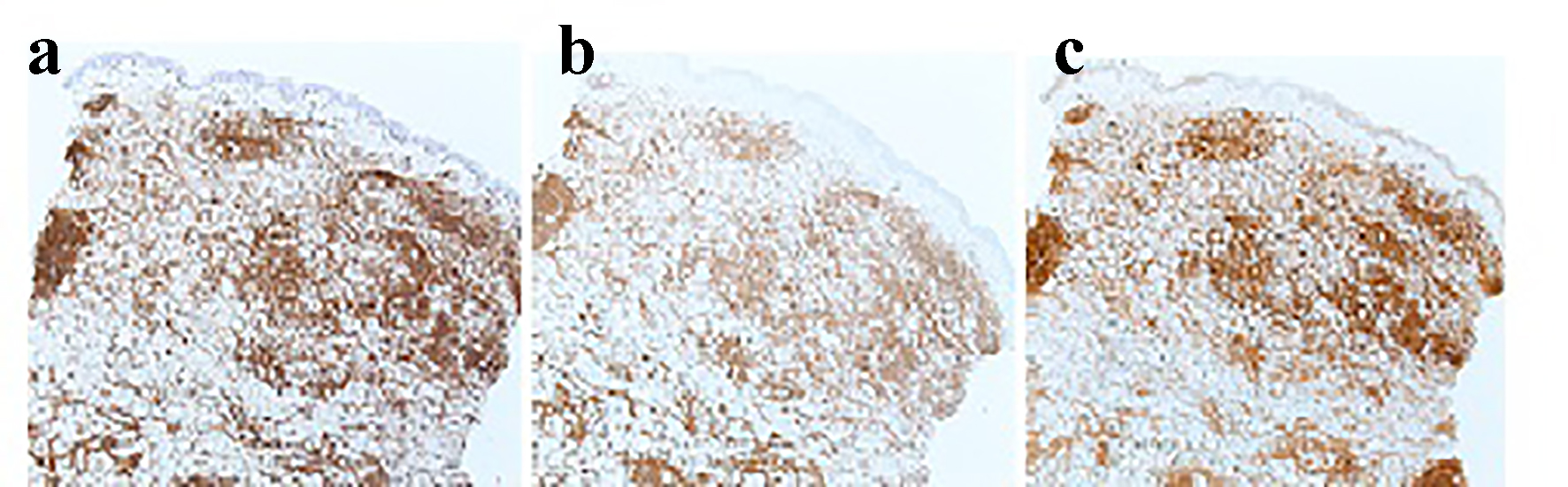 Click for large image | Figure 2. Immunostain of skin biopsy: (a) expression of CD5 by neoplastic cells (× 4); (b) expression of CD20 (× 4); (c) expression of cyclin D1 by the malignant lymphoid cells (× 4). |
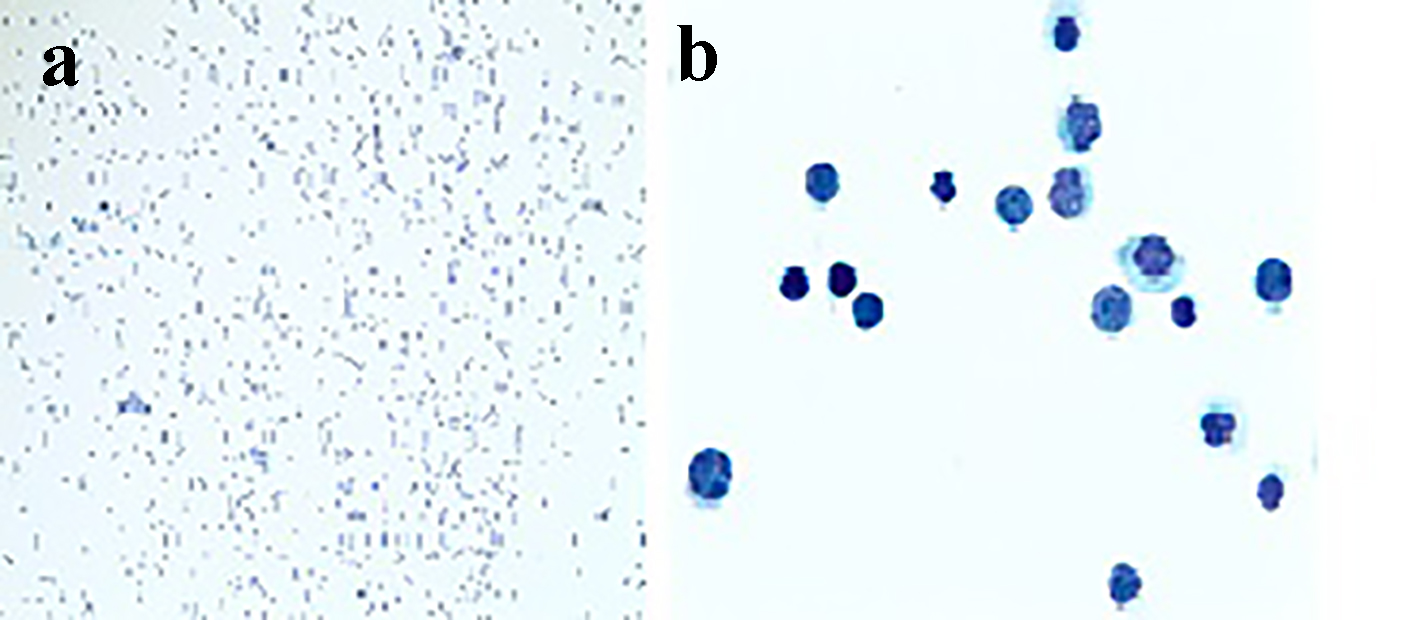 Click for large image | Figure 3. CSF cytology with × 10 and × 100 magnifications in (a) and (b), respectively, showing cells suspicious for lymphoma which stained positive for cyclin D1 immunostain. |
At the time of relapse, she continued to be in complete hematologic remission with no evidence of adenopathy or splenomegaly on imaging and completely normal counts.
The patient was immediately started on intrathecal cytarabine and methotrexate (initially twice a week, then weekly) along with ibrutinib. She was also initiated R-CHOP for the MCL relapse. Repeat CSF cytology done after 10 days of initiation of chemotherapy did not detect any lymphoma cells. Her skin lesions completely resolved and her visual symptoms have improved as well in 2 weeks. Patient is currently continuing on the above stated treatment.
| Discussion | ▴Top |
MCL exists in four cytogenetic variants: blastoid, pleomorphic, small-cell and marginal zone-like. Among these, blastoid and pleomorphic variants have been generally associated with morbid prognosis and incurability [7]. The blastoid variant is found in 10-30% of those diagnosed with MCL and has a shorter median survival time than classical type (15 vs. 45 months) [5, 8, 9]. The skin involvement, even if rare, has been more frequently reported in the blastoid variant of MCL [2]. MCL appears as monomorphic, small-to-medium-sized lymphoid cells with irregular nuclear contours on microscopy [10], while the blastoid variant exhibits lymphoblast-like cells with dispersed chromatin and high mitotic rate (approximately 20 - 30/high power field (HPF)) [5].
Immunophenotyping is an important component of the diagnosis, which involves testing for the characteristic translocation (11:14), which causes a juxtaposition of the CCND-1 gene, a gene that encodes for cyclin D1 on chromosome 11 with the immunoglobulin heavy chain gene on chromosome 14 [1]. This results in overexpression of cyclin D1, which serves as a positive signal for transition to the S phase [2]. Similar to translocation (11:14), cyclin D1 is characteristic to MCL and is expressed in more than 60-70% of cases of MCL but also can be positive in 4% of B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (B-CLL/SLL) [11]. The lymphoma is usually positive for B-cell markers, like CD79a, CD19, CD20, CD22 and CD5 as well as usually negative for CD10, CD23 and BCL-6 [9]. CD5 immunoreactivity helps differentiate MCL from other B-cell lymphomas such as follicular or marginal zone lymphomas but it can be absent in 10-30% of all MCL cases. In such instances, cyclin D1 testing by fluorescence in situ hybridization can help diagnose MCL [3, 12]. Rarely cyclin D1 and the (11; 14) translocation can also be absent in MCL, in which case SOX 11, a novel marker reported to be present in 90% of MCLs and in all cyclin D1-negative MCLs can be helpful [4, 13].
While involvement of extranodal organs is common in MCL, it is most frequently found in bone marrow and gastrointestinal tract, while MCL in skin or orbit is quite rare and almost always found in disseminated disease, comprising around 17% of patients with IV stage disease. Upon review of the literature, only 24 case reports of MCL affecting skin were found, of which 19 patients had stage IV disease with involvement of the lymph nodes and the bone marrow at the time of diagnosis. Six of the 24 patients involved skin at a relapse with no evidence of systemic involvement, most presenting 6 months to 3 years after the diagnosis [14].
Most commonly affected areas were the trunk followed by the face, arms, legs, thighs and the abdomen [8, 9]. Commonly observed skin lesions include nodules, macules, tumoral plaques and subcutaneous infiltrations [8, 14]. The average age of the patients with cutaneous MCL was 64.4 years (range: 22 - 89 years), with male to female ratio of 19: 5 [14].
Patients who developed skin lesions with widespread MCL typically had a poor prognosis with high mortality despite aggressive chemotherapy. Of the 24 cases, 11 patients achieved a complete remission and no recurrence was observed during a follow-up period [14].
When MCL involves the eye, it is most commonly found in orbit and ocular adnexa [10]. When a malignancy occurs at these sites, it is most likely to be a B-cell non-Hodgkin’s lymphoma (NHL) than any other type. Lymphomas at the orbit or ocular adnexa constitute around 1-2% of lymphomas and about 8% of the extranodal lymphomas [15-17]. Marginal zone lymphoma comprises approximately 80-90% of the NHLs in these areas [15, 18, 19]. Less common types include follicular lymphoma, diffuse large B-cell lymphoma, plasmacytoma, lymphoplasmacytic lymphoma [15, 18] and MCL, the latter constituting of only 1-9% of all lymphomas in the region [10, 12].
Due to the aggressive nature of MCL and usual presentation at an advanced stage, prognosis is already poor and there is no curative treatment. For the treatment of MCL, a combination of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) had been a standard of care for a long time until regimens such as bendamustine and rituximab (BR) were tried and started being preferred due to favorable side effect profile and superior progression-free survival (PFS) compared to R-CHOP [20]. Aggressive induction regimens such as rituximab plus cyclophosphamide, doxorubicin, vincristine, and dexamethasone alternating with high-dose cytarabine and methotrexate (R-HyperCVAD) and the Nordic regimen using high-dose R-CHOP alternating with rituximab plus high- dose cytarabine have been preferred in young, healthy patients [21, 22]. Stem cell transplant is usually preferred after the consolidation chemotherapy since it has demonstrated a prolonged disease-free survival [23, 24].
Various systemic and local chemotherapeutic strategies have been employed in MCL that secondarily involves eye and skin. Not many treatment options have shown promising results but the commonly used regimens include cyclophosphamide, vincristine, prednisone (CVP); cyclophosphamide, hydroxyl-daunorubicin, vincristine, prednisone (CHOP); and mitoxantrone, chlorambucil, and prednisone [25]. As evident by the Rasmussen study, survival was better in patients receiving rituximab in combination with other regimens than those without rituximab likely secondary to its immune effect, that is anti-CD20 activity (5-year overall survival (OS): 83% versus 8%) [10]. Bone marrow transplantation has also shown promising results in some cases [10, 12]. Proteasome inhibitors (bortezomib), mTOR inhibitors (temsirolimus), and immunomodulatory drugs (lenalidomide) are other treatment options with growing use in MCL, especially for patients who have failed first-line regimens [26]. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, was recently introduced for use in those who had received at least one prior therapy and present with a relapse [27]. However, the role of these regimens in skin and ocular MCL is yet to be evaluated.
When MCL involves the eye, the patient usually has an established diagnosis of the lymphoma and in the rare cases when it is primary it is almost always stage IV [16]. In a study performed by Nola et al, median PFS was reported to be 12 months, with a median OS of 57 months, with almost all cases complicated by recurrences [18].
Additional markers of poor prognosis in ocular MCL include increased mitotic activity (> 20 mitoses/HPF), involvement of other organs and genetic changes such as the P53 mutation and deletion of P16 [6, 12]. Moreover, Rasmussen et al expressed that patients with primary ocular MCL had better survival compared to patients with involvement of orbit after an established diagnosis of MCL (51 months vs. 43 months), likely secondary to the bone marrow involvement which was more common in the latter group of patients [10].
In a study, five of 22 patients (22%) developed CNS infiltration of MCL during a follow-up period of 7 years. All of these patients presented with poor histologic subtypes and advanced disease, with most presenting as resistant disease or relapse with disseminated disease [28].
In our case, we had skin, CNS and ocular involvement at relapse. Given that ibrutinib can penetrate the blood-brain barrier, it was used in combination with R-CHOP to help with initial cytoreduction along with intrathecal treatment for her CNS disease. A good initial response was seen in terms of resolution of her skin lesions, negative CSF cytology and improvement in her visual symptoms.
Conclusion
MCL is a rapidly progressing lymphoma which when it involves skin, orbit or CNS, suggests disseminated disease. Involvement of all three of these organs, although rare, significantly worsens the outcome. While our patient had prior diagnosis of the lymphoma, a high suspicion was necessary to look for rare presentations of this disease relapse. Treatment for MCL is evolving, which further limits options when it involves extranodal sites and these less common organs. Randomized trials need to be done to evaluate specific therapy for uncommon presentations such as the one we have described.
| References | ▴Top |
- Seto M, Yamamoto K, Iida S, Akao Y, Utsumi KR, Kubonishi I, Miyoshi I, et al. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992;7(7):1401-1406.
pubmed - Canpolat F, Tas E, Albayrak Sonmez A, Oktay M, Eskioglu F, Alper M. Cutaneous presentation of mantle cell lymphoma. Acta Derm Venereol. 2010;90(5):548-550.
doi pubmed - Liu Z, Dong HY, Gorczyca W, Tsang P, Cohen P, Stephenson CF, Berger CS, et al. CD5- mantle cell lymphoma. Am J Clin Pathol. 2002;118(2):216-224.
doi pubmed - Mozos A, Royo C, Hartmann E, De Jong D, Baro C, Valera A, Fu K, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94(11):1555-1562.
doi pubmed - Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89(6):2067-2078.
pubmed - Vose JM. Mantle cell lymphoma: 2013 Update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2013;88(12):1082-1088.
doi pubmed - Swerlow SH, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. p. 89.
- Wehkamp U, Pott C, Unterhalt M, Koch K, Weichenthal M, Klapper W, Oschlies I. Skin Involvement of Mantle Cell Lymphoma May Mimic Primary Cutaneous Diffuse Large B-cell Lymphoma, Leg Type. Am J Surg Pathol. 2015;39(8):1093-1101.
doi pubmed - Estrozi B, Sanches JA, Jr., Varela PC, Bacchi CE. Primary cutaneous blastoid mantle cell lymphoma-case report. Am J Dermatopathol. 2009;31(4):398-400.
doi pubmed - Rasmussen P, Sjo LD, Prause JU, Ralfkiaer E, Heegaard S. Mantle cell lymphoma in the orbital and adnexal region. Br J Ophthalmol. 2009;93(8):1047-1051.
doi pubmed - Asaad NY, Abd El-Wahed MM, Dawoud MM. Diagnosis and prognosis of B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (B-CLL/SLL) and mantle cell lymphoma (MCL). J Egypt Natl Canc Inst. 2005;17(4):279-290.
pubmed - Looi A, Gascoyne RD, Chhanabhai M, Connors JM, Rootman J, White VA. Mantle cell lymphoma in the ocular adnexal region. Ophthalmology. 2005;112(1):114-119.
doi pubmed - Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3(2):185-197.
doi - Kalinska-Bienias A, Ziarkiewicz-Wroblewska B, Kowalewski C, Wozniak K. Mantle cell lymphoma with skin involvement. Postepy Dermatol Alergol. 2015;32(3):229-234.
doi pubmed - Coupland SE, Krause L, Delecluse HJ, Anagnostopoulos I, Foss HD, Hummel M, Bornfeld N, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998;105(8):1430-1441.
doi - Ferry JA, Fung CY, Zukerberg L, Lucarelli MJ, Hasserjian RP, Preffer FI, Harris NL. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31(2):170-184.
doi pubmed - Rootman DB, Mavrikakis I, Connors JM, Rootman J. Primary, unilateral ocular adnexal lymphoma: disease progression and long-term survival. Ophthal Plast Reconstr Surg. 2011;27(6):405-409.
doi pubmed - Nola M, Lukenda A, Bollmann M, Kalauz M, Petrovecki M, Bollmann R. Outcome and prognostic factors in ocular adnexal lymphoma. Croat Med J. 2004;45(3):328-332.
pubmed - Yoo SB, Kim YA, Jeon YK, Kim CW. CD5-undetected by immunohistochemistry, t(11;14)(q13;q32)-positive conjunctival mantle cell lymphoma: a case report. Pathol Res Pract. 2008;204(10):779-783.
doi pubmed - Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210.
doi - Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013-7023.
doi pubmed - Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, Pedersen LB, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158(3):355-362.
doi pubmed - Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, Metzner B, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677-2684.
doi pubmed - LaCasce AS, Vandergrift JL, Rodriguez MA, Abel GA, Crosby AL, Czuczman MS, Nademanee AP, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: an analysis from the NCCN NHL Database. Blood. 2012;119(9):2093-2099.
doi pubmed - Hatef E, Roberts D, McLaughlin P, Pro B, Esmaeli B. Prevalence and nature of systemic involvement and stage at initial examination in patients with orbital and ocular adnexal lymphoma. Arch Ophthalmol. 2007;125(12):1663-1667.
doi pubmed - Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26-38.
doi pubmed - Herrera AF, Jacobsen ED. Ibrutinib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2014;20(21):5365-5371.
doi pubmed - Montserrat E, Bosch F, Lopez-Guillermo A, Graus F, Terol MJ, Campo E, Rozman C. CNS involvement in mantle-cell lymphoma. J Clin Oncol. 1996;14(3):941-944.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


