| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 2, May 2018, pages 83-85
Pegloticase Induced Hemolytic Anemia in a Patient With G6PD Deficiency
Michael L. Adasheka, b, Khalil I. Bourjia
aDepartment of Internal Medicine, Sinai Hospital, Baltimore, MD, USA
bCorresponding Author: Michael L. Adashek, Department of Internal Medicine, Sinai Hospital, 2401 W. Belvedere Ave., Baltimore, MD 21215, USA
Manuscript submitted March 18, 2018, accepted April 20, 2018
Short title: Pegloticase Induced Hemolytic Anemia
doi: https://doi.org/10.14740/jh402w
| Abstract | ▴Top |
Gout is a metabolic disorder of purine metabolism that results in crystallization of uric acid in the form of monosodium urate crystals, affects 8.3 million Americans and is the most common cause of inflammatory arthritis in adults. Urate lowering therapy is the mainstay of treatment for chronic gout. Initial treatments of choice in gout include allopurinol, a purine analog which inhibits xanthine oxidase and decreases the production of uric acid as well as probenecid which increases the urinary excretion of uric acid. However, 3% of patients will fail these treatments. In 2010, pegloticase, a recombinant urate oxidase conjugated to polyethylene glycol, was approved for these patients. Pegloticase has been shown to rapidly normalize plasma uric acid values, resolve tophi and improve quality of life in these patients. Hereby we present a case of a 50-year-old African male admitted to the hospital with symptomatic anemia 1 week after pegloticase infusion. He was found to have glucose-6-phosphate dehydrogenase deficiency, predisposing him to hemolytic anemia. Hereby we discuss his clinical course, and suggest glucose-6-phosphate dehydrogenase deficiency screening prior to pegloticase infusion.
Keywords: Refractory gout; Pegloticase; Hemolytic anemia; G6PD deficiency
| Introduction | ▴Top |
Pegloticase is a recombinant urate oxidase conjugated to polyethylene glycol (PEG) that is approved for management of chronic refractory gout after failure of conventional urate-lowering therapies (ULTs) (i.e., allopurinol, febuxostat or probenecid) [1]. Although pegloticase has been shown to rapidly normalize plasma uric acid (PUA) values, resolve tophi and improve quality of life and function in these patients, a thorough understanding of its adverse reactions is lacking. We hereby present a case of hemolytic anemia induced by pegloticase in patient with glucose-6-phosphate dehydrogenase (G6PD) deficiency [2-4].
| Case Report | ▴Top |
A 50-year-old African American male with a medical history of hypertension, gout and rheumatoid arthritis presented to outpatient clinic complaining progressively worsening dyspnea over a course of a week. The patient was found to have an oxygen saturation of 92% with concomitant anemia and was immediately referred to the emergency department (ED). In the ED, the patient endorsed intermittent dyspnea and complained of “tea colored urine” that had begun approximately 2 days after starting a new medication for his uncontrolled gout, pegloticase. His home medications included amlodipine (10 mg/day), aspirin (81 mg/day), carvedilol (3.125 mg/day) and methotrexate (10 mg/week). He was then admitted to the general medicine ward for further evaluation and management of his anemia.
On examination, the patient’s oxygen saturation was 92% on room air and other vital signs were within normal limits. His jugular venous pressure waveform was not elevated, and his cardiopulmonary examination was normal. No hepatosplenomegaly was appreciated, nor abdominal or suprapubic tenderness. He had no lower extremity edema and no stigmata suggestive of chronic liver disease. The neurological examination was normal. His initial labs were significant for hyponatremia (132 mmol/L), elevated blood urea nitrogen (BUN) (25 mmol/L), normal creatinine (0.85 mg/dL), elevated direct bilirubin (0.9 mg/dL) and total bilirubin (4.2 mg/dL), as was the patient’s lactate dehydrogenase (876 U/L). The patient’s complete blood count revealed leukocytosis (16.9 × 103/mm3), and macrocytic anemia with a hemoglobin of 7.3 g/dL, hematocrit of 24.2%, mean corpuscular volume of 101.7 fL, and red cell distribution width was elevated at 16%. He did have an elevated D-dimer (3.1 mg/L) and fibrinogen activity (523 mg/dL) with normal coagulation studies. Further testing showed low haptoglobin (17 mg/dL), and normal vitamin B12 and folate levels. Urinalysis was remarkable for a large amount of hemoglobin, 2+ protein and 2+ urobilogen with < 1 red blood cell per high powered field and the urine myoglobin was significantly elevated at 814 ng/mL.
A computerized tomography (CT) scan of the abdomen and pelvis with IV contrast was obtained which revealed colonic diverticulosis, hepatic steatosis and avascular necrosis of the femoral heads. A chest CT with IV contrast did not show any signs of pulmonary embolism. To better define this presumable hemolytic anemia, further tests were done and revealed normal red blood cell folate hemolysate and creatine phosphokinase, negative direct Coombs test and peripheral blood smear that failed to show significant anisopoikilocytosis or abnormal red blood cell (Fig. 1). However, quantitative G6PD was 194, and red blood cell G6PD was low at 2.14 × 106 (normal range: 4.14 × 106 - 5.80 × 106), confirming a diagnosis of hemolytic anemia with G6PD deficiency.
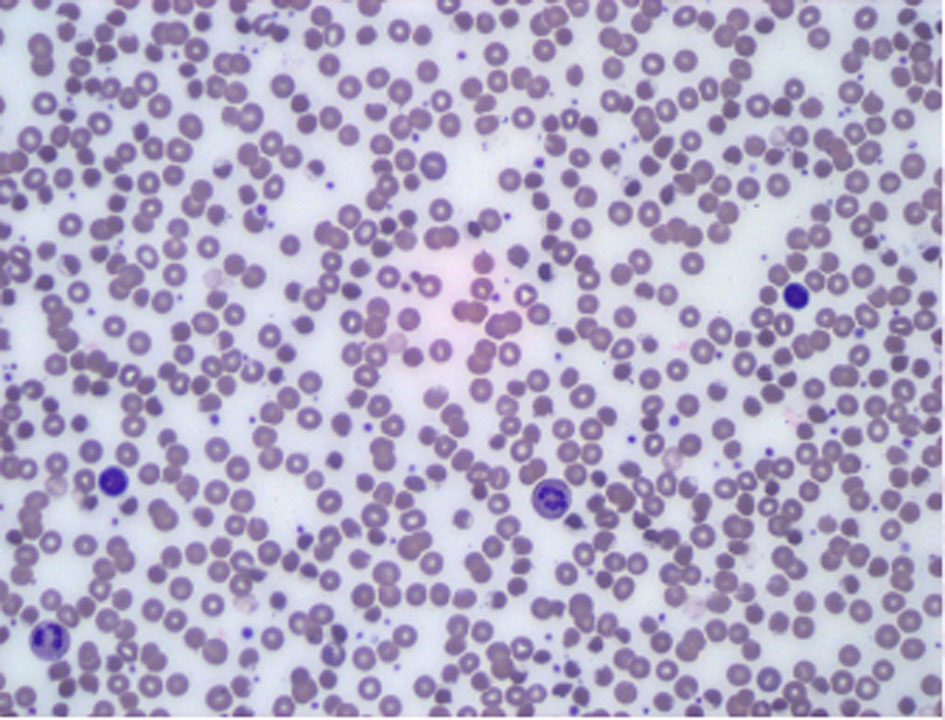 Click for large image | Figure 1. Peripheral blood smear. Increased white cell population (mostly granulocytes-mature neutrophils). Background lymphocytes and monocytes are morphologically unremarkable. Erythroid elements show no significant anisopoikilocytosis. There is no significant population of specific abnormal forms such as teardrop cells, schistocytes or target cells. |
Patient was managed with IV hydration (which resolved both the patient’s hyponatremia and elevated BUN) and required transfusion of 3 packed RBC units. After 4 days spent on the medicine ward, patient’s symptoms resolved, and the patient was discharged home.
| Discussion | ▴Top |
To our knowledge, this is the third case of hemolytic anemia triggered by pegloticase in patients with G6PD deficiency [5, 6]. Pegloticase actively converts urate to allantoin, a more soluble end product of purine metabolism. During this process hydrogen peroxide is generated and creates an oxidative insult that triggers hemolysis in predisposed patients, such as those with G6PD deficiency. Patients with G6PD deficiency were excluded from initial trials of pegloticase due to a potential predisposition for hemolysis under oxidative stressors. G6PD is the only source of reduced glutathione in a red blood cell that mediates oxidative stress. This enzymatic deficiency affects approximately 400 million people worldwide [7]. In the USA, epidemiological studies have suggested that as many as 13% of African American males possess G6PD deficiency [8]. Therefore, we recommend screening for G6PD deficiency prior to pegloticase initiation. Finally, if a patient experience hemolytic anemia after pegloticase infusion, supportive treatment with red blood cell infusions should be initiated and plasmapheresis may be required given pegloticase’s 14-day half-life [9].
Conflict of Interest
None of the authors have any financial or personal bias to declare.
Informed Consent
Informed consent was obtained from the patient for educational use of the below mentioned data and no personal patient information has been disclosed. This paper has been written in keeping with the principles of the Declaration of Helsinki.
Author Contributions
Both authors have contributed equally to the drafting and production of this manuscript.
| References | ▴Top |
- Sundy JS, Becker MA, Baraf HS, Barkhuizen A, Moreland LW, Huang W, Waltrip RW, 2nd, et al. Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum. 2008;58(9):2882-2891.
doi pubmed - Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, Vazquez-Mellado J, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306(7):711-720.
doi pubmed - Strand V, Khanna D, Singh JA, Forsythe A, Edwards NL. Improved health-related quality of life and physical function in patients with refractory chronic gout following treatment with pegloticase: evidence from phase III randomized controlled trials. J Rheumatol. 2012;39(7):1450-1457.
doi pubmed - Araujo EG, Bayat S, Petsch C, Englbrecht M, Faustini F, Kleyer A, Hueber AJ, et al. Tophus resolution with pegloticase: a prospective dual-energy CT study. RMD Open. 2015;1(1):e000075.
doi pubmed - Owens RE, Swanson H, Twilla JD. Hemolytic Anemia Induced by Pegloticase Infusion in a Patient With G6PD Deficiency. J Clin Rheumatol. 2016;22(2):97-98.
doi pubmed - Geraldino-Pardilla L, Sung D, Xu JZ, Shirazi M, Hod EA, Francis RO. Methaemoglobinaemia and haemolysis following pegloticase infusion for refractory gout in a patient with a falsely negative glucose-6-phosphate dehydrogenase deficiency result. Rheumatology (Oxford). 2014;53(12):2310-2311.
doi pubmed - Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 1991;324(3):169-174.
doi pubmed - Burka ER, Weaver Z, 3rd, Marks PA. Clinical spectrum of hemolytic anemia associated with glucose-6-phosphate dehydrogenase deficiency. Ann Intern Med. 1966;64(4):817-825.
doi pubmed - Krystexxa [package insert]. Glendale, WI: Crealta parmaceuticals; 2010.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


