| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 3, September 2018, pages 128-130
In Situ Leukemia as Manifestation of Relapse After Allogeneic Transplantation of Bone Marrow of ALL B: A Case Report
Irline Cordeiro de Macedo Pontesa, c, Marjory Medeiro Passos Teixeiraa, Naisa Bezerra de Carvalhoa, Milton da Silva Linhares Juniora, Luis Fabio Barbosa Botelhob
aUniversidade Federal da Paraiba (UFPB), Hospital Universitario Lauro Wanderley (HULW), Joao Pessoa-PB, Brazil
bHematology Unit, Department of Internal Medicine, Universidade Federal da Paraiba (UFPB), Hospital Universitario Lauro Wanderley (HULW), Joao Pessoa-PB, Brazil
cCorresponding Author: Irline Cordeiro de Macedo Pontes, Universidade Federal da Paraiba (UFPB), Hospital Universitario Lauro Wanderley (HULW), Joao Pessoa-PB, Brazil
Manuscript submitted June 18, 2018, accepted July 30, 2018
Short title: In Situ Leukemia as Relapse of ALL B
doi: https://doi.org/10.14740/jh436w
| Abstract | ▴Top |
Acute lymphoid leukemia (ALL) is a malignant proliferation of abnormal lymphoid precursors in the bone marrow. It is the most common acute leukemia in childhood, accounting for only 20% of acute leukemia in adults. The clinical presentation is heterogeneous including pallor, fatigue and weakness. Diagnosis is performed through myelogram, bone marrow biopsy and immunophenotyping. Chemotherapy and, in some cases, bone marrow transplantation (BMT) are used to treat the disease. However, the efficacy of treatment in adult patients is still below expected and therapeutic innovations have not increased disease-free survival. In addition, about 20% of patients have relapse, usually generalized, of the disease. This article aims to report the case of a patient previously treated for ALL B presenting localized medullar relapse or in situ leukemia.
Keywords: Precursor cell lymphoblastic leukemia-lymphoma; Therapeutics; Recurrence
| Introduction | ▴Top |
The acute lymphoid leukemia (ALL) is a malignant lymphoproliferative disease with heterogeneous clinical presentation. Immature cells lose the capacity of differentiate and infiltrate the bone narrow leading to a suppression of normal hematopoiesis. It is the most common acute leukemia in children, accounting for only 20% of acute leukemia in adults [1]. The diagnosis is based on bone marrow aspiration, bone marrow biopsy and immunophenotyping by flow cytometry. The symptoms more commonly found are pallor, fatigue and weakness. Nocturnal hyperhidrosis and hemorrhagic manifestations are seen in 33% of the patients [2].
Adults have a poor prognosis. One of the determinants of prognosis is genetics: hyperploidia and t(12; 21) indicate good prognosis, but are characteristic of children and adolescents, being rare in adults. The translocation between the long arms of chromosomes 9 and 22 generating the BCR-ABL gene (the Philadelphia chromosome) implies poor prognosis and are more common in adults. Another important prognostic factor is the minimum residual disease (MRD). It evaluates the response of induction through flow cytometry or other methods. It can be performed at various times of treatment, but the post-induction MRD is the most important prognostic factor [3].
The treatment is based on chemotherapy performed in four stages: induction chemotherapy, consolidation therapy, central nervous system prophylaxis and maintenance [4]. In some cases, hematopoietic stem cell transplantation (HSCT) can be performed. However, treatment of adult patients remains unsatisfactory and therapeutic innovations have not increased disease-free survival rates in the last two decades: adults have a 5-year survival rate of only 30% [3].
| Case Report | ▴Top |
A 31-year-old female with history of Pre B ALL treated with hyper-CVAD chemotherapy and allogeneic bone marrow transplantation (BMT) from a compatible related male donor in the first remission. The cytogenetic study was performed at diagnosis, but molecular test for BCR/ABL translocations in blood samples was negative. The transplantation was performed in another institution in a referring center, far away from her home city. It was used a conditioning treatment with busulfan 16 mg/kg, orally, for 4 days and cyclophosphamide 120 mg/kg, IV, for 2 days and prophylaxis of graft versus host disease (GVHD) with cyclosporine and methotrexate for 6 months after HSCT. The cell source was peripheral stem cell. There are scarce data about this period of treatment.
Three years after the transplantation, already in no use of immunosuppressors nor showing any sign of GVHD, the patient presented with pain in both feet. The pain was more intense in ankle and it did not improve with analgesics. Due to suspicion of recurrent disease, a bone marrow aspirate with immunophenotyping was performed, but no alterations were detected.
In face of the persistence and progression of the pain, a nuclear magnetic resonance imaging (MRI) of both feet was performed, revealing non-expansive, permeation-like lesions in the bone marrow of various limb bones (Fig. 1). Since there was high probability of relapse of the disease, another bone marrow aspirate in iliac crest with immunophenotyping and a bone marrow biopsy were performed. The results, however, did not indicate activity of the disease. The chimerism analysis showed donor complete chimera. Lactate dehydrogenase (LDH) and cerebrospinal fluid (CSF) were unremarkable.
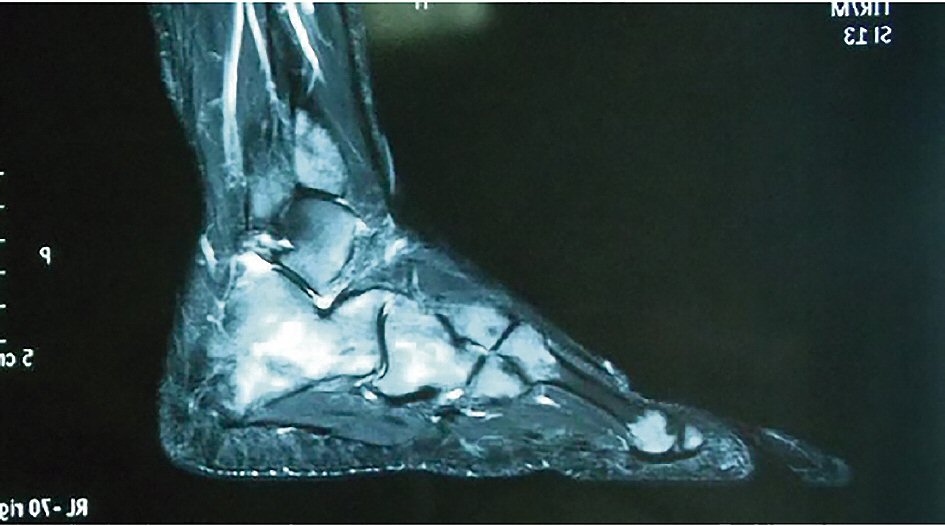 Click for large image | Figure 1. Nuclear magnetic resonance imaging of the foot. |
Due to the persistence of pain and MRI findings, multiple biopsies of the lesions were performed, which were compatible with lymphoblastic non-Hodgkin’s lymphoma type B/ALL, with exclusive bone marrow involvement (Fig. 2). The immunohistochemistry study was positive for the following markers: CD19, CD79a, CD10, CD20, CD34 and TdT. These markers were negative: CD3, CD56, CD33 and CD13. No molecular or cytogenetics analysis was done in the material mostly because paucity of these exams in our city.
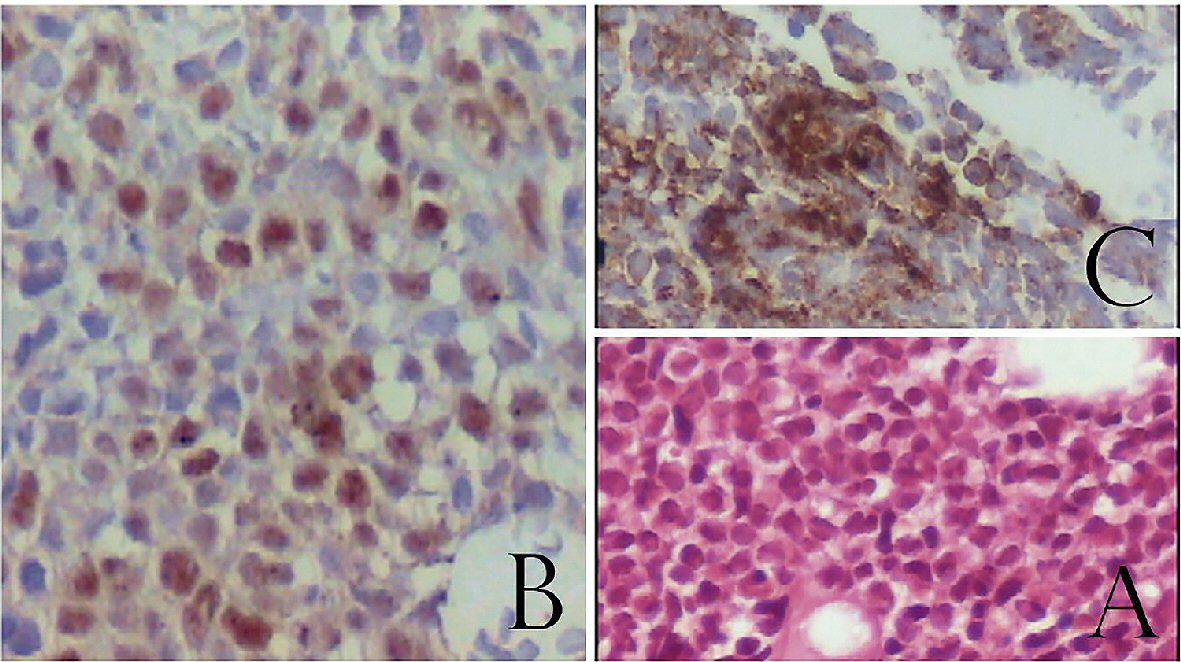 Click for large image | Figure 2. Bone marrow biopsy. (A) H&E staining showing infiltration by blast cells. (B) TdT positive cells confirming immature lymphoid cells. (C) CD20 positive cells confirming B cell ontogeny. |
Because of two bone marrow aspirates with immunophenotyping and an iliac crest biopsy presented with no alterations, the finding was interpreted as localized marrow relapse or “in situ” leukemia.
The patient was again referred to a specialized center in another city and received donor lymphocyte infusion (DLI). However, after 4 months of treatment, the patient died due to infections complications. There was not enough information regarding her last treatment.
| Discussion | ▴Top |
The results obtained from the treatment of adult patients with ALL are significantly inferior to those observed in children. Only 25% of adult patients achieve a 5-year disease-free survival and long-term survival is obtained in 38% of the patients [5]. Treatment of ALL can be performed with a chemotherapy regimen and HSCT [2].
Although there are studies and meta-analysis comparing chemotherapy and HSCT [6], indications of transplantation in adults in first remission are not accurate and require continuous updating [5]. However, risk stratification and minimal residual studies can be helpful to define the patient who benefits most from the transplant.
Allogeneic transplantation has been shown to be effective in adult patients with high-risk ALL. Transplant benefit was reported in young adult patients with severe disease in first remission or patients in second remission. The transplantation from a related compatible donor was the best option [1].
In addition, the importance of this therapy has already been demonstrated in non-severe patients with a living related donor, raising the question if it is the best option for patients in first remission [7]. Those studies do not address the access of the population to all treatments available. In Brazil, the BMT is a good option for patients who do not have entirely access to new drug, clinical trial or even the full diagnostic studies like our patient.
About 20% of the patients present with relapse [8], commonly affecting the bone marrow and, potentially, any tissue in the body, particularly the gonads and the nervous system. In these cases, the bone marrow aspirate is a good tool to detect the relapse. Relapses isolated in other organ except bone marrow are rare, and are called lymphoblastic lymphomas [9]. Our patient did not have hematologic relapse detected with the classical methods.
In the case report, relapse occurred only in the bone marrow of the foot bones. The myelogram and immunophenotyping of the sternum and iliac crest did not present with any alterations, delaying the diagnosis. The disease was only identified by an imaging exam which demonstrated a lesion leading to a biopsy to confirm the diagnosis. Relapse in situ as described above is extremely rare. As far as we know, this is the first case reported in the literature with this pattern of “in situ” relapse after HSCT. Although the “in situ” relapse was seen before systemic disease appears, the outcome was dismal as predict, despite the treatment. Therefore, in patients with ALL and localized bone pain of obscure origin, a normal myelogram should not initially rule out the possibility of relapse.
Conclusions
ALL in adults is very difficult to cure. BMT is a good option in some cases, but relapses, even in uncommon sites, still remains a challenge. Clinical suspicious and monitoring are, maybe, the best weapons to detect an early relapse that can be treated as soon as possible. Even little symptoms must alert the physician to seek beyond the obvious.
Acknowledgments
Special acknowledgment to the Centro de Ciencias Medicas (CCM) of the Universidade Federal da Paraiba (UFPB) and to the Hospital Universitario Lauro Wanderley (HULW), which always provided an environment of learning, search for knowledge and scientific discussions.
Conflict of Interest
None.
Funding
There was no funding source.
| References | ▴Top |
- Zanichelli MA, Colturato VR, Sobrinho J. Indicacoes em transplante de celulas-tronco hematopoeticas em pacientes adultos com leucemia linfoide aguda. Rev Bras Hematol Hemoter. 2010;32:54-60.
doi - Zago MA, Falcao RP, Pasquini R. Tratado de Hematologia. 1a. Rio de Janeiro; 2012. p. 899.
- Spinelli O, Tosi M, Peruta B, Guinea Montalvo ML, Maino E, Scattolin AM, Parolini M, et al. Prognostic significance and treatment implications of minimal residual disease studies in Philadelphia-negative adult acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2014;6(1):e2014062.
doi pubmed - Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166-178.
doi pubmed - Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C, Committee EG. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69-v82.
doi - Nishiwaki S, Miyamura K, Ohashi K, Kurokawa M, Taniguchi S, Fukuda T, Ikegame K, et al. Impact of a donor source on adult Philadelphia chromosome-negative acute lymphoblastic leukemia: a retrospective analysis from the Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Ann Oncol. 2013;24(6):1594-1602.
doi pubmed - Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833.
doi pubmed - Stein A, Forman SJ. Allogeneic transplantation for ALL in adults. Bone Marrow Transplant. 2008;41(5):439-446.
doi pubmed - Brito AC, Capistrano HM, Torres ML, Ramos G, Viana MB, de Oliveira BM. Isolated relapse in the oral cavity of a child with T-lineage acute lymphoblastic leukemia. Braz Dent J. 2012;23(6):711-715.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


