| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 1, March 2019, pages 34-36
Tumor Thrombus From Urothelial Carcinoma of Renal Pelvis
Kavya Kannana, b, Aysha Amjada, Yu Sunny Yanga, Abhishek Duttaa, Barbara Pisania
aWake Forest Baptist Medical Center, Winston-Salem, NC, USA
bCorresponding Author: Kavya Kannan, Wake Forest Baptist Medical Center, Winston-Salem, NC 27157, USA
Manuscript submitted December 6, 2018, accepted February 8, 2019
Short title: Tumor Thrombus From Urothelial Carcinoma
doi: https://doi.org/10.14740/jh472
| Abstract | ▴Top |
We present a case report of an unfortunate 67-year-old man. He developed symptoms of thromboembolic disease in the immediate post-operative period after nephroureterectomy with recurrent pulmonary embolism in spite of being on several different anticoagulation regimens. He was later found to have tumor thrombus in the pulmonary artery secondary to urothelial tumor extension.
Keywords: Pulmonary embolus; Tumor thrombus; Anticoagulation; Urothelial carcinoma; Thrombo-embolectomy
| Introduction | ▴Top |
Tumor thrombus, oftentimes, is a clinical diagnosis with no supporting evidence to sway our decision on the management, due to the need for an invasive testing/biopsy to prove the same. This diagnosis needs a high clinical suspicion and becomes important, especially in a patient who is on cancer surveillance, and oftentimes, has significant prognostic implications. Our case report focuses on the utility of having such high suspicion and its contribution to alter treatment course.
| Case Report | ▴Top |
A 67-year-old man presented to the emergency department with syncope.
Seven months prior to this presentation, he was diagnosed with pT3 NX M0 stage III high-grade urothelial carcinoma of the right renal pelvis with peri-neural and peri-vascular invasion. He underwent laparoscopic right nephro-ureterectomy with bladder cuff. Four days after surgery, patient presented to the emergency room with acute severe shortness of breath and hypotension that responded to fluids. Computed tomography angiography (CTA) of the chest revealed extensive filling defects demonstrated in both the distal main pulmonary arteries extending into multiple bilateral upper and lower segmental pulmonary arteries, and low likelihood of right heart strain. Bilateral lower extremity Doppler ultrasonography revealed no evidence of deep vein thrombosis (DVT) in lower extremities. He was started on systemic anticoagulation followed by warfarin bridge. Patient was relatively well until 2 months later, when he developed another episode of acute severe shortness of breath. He was then diagnosed to have new pulmonary embolism while being on warfarin therapy. Patient elected to have inferior vena cava (IVC) filters placed even though there was no Doppler evidence for DVT and anticoagulation was transitioned from warfarin to rivaroxaban. His echo at that time revealed mild pulmonary hypertension without evidence of right heart strain. From the cancer perspective, he was considered to have no evidence of residual disease based on imaging and plan was to manage conservatively with follow-up imaging in 3 months due to intermediate to high risk of recurrence. Patient is a former smoker and quit 20 years prior to this presentation. His brother died of bladder cancer at the age of 50.
Patient presented with another episode of shortness of breath 1 month after starting rivaroxaban. His CTA showed new pulmonary embolus in the right main pulmonary artery with evidence of right heart strain. Due to recurrent pulmonary emboli, his anticoagulation was changed to fondaparinux and aspirin of 325 mg. Patient unfortunately presented 1 month later with worsening shortness of breath, palpitations with electrocardiogram (EKG) evidence of sinus tachycardia and hypotension that responded to fluids. CTA (Fig. 1) showed extensive occlusive pulmonary emboli involving most of the branches of left main pulmonary artery and most of the branches of right lower lobe pulmonary artery with echo evidence of significant right heart strain, biventricular failure with left ventricular ejection fraction of 40%, moderate to severe pulmonary hypertension, and moderate tricuspid regurgitation. Anticoagulation was transitioned to lovenox and anti-factor Xa level remained normal during that hospitalization. Interventional radiology and cardiothoracic surgery planned for invasive intervention only if he fails a trail of lovenox and was discharged as he remained hemodynamically stable during that hospitalization. Thrombolytic therapy was deferred due to his hemodynamic stability. For new biventricular failure, patient was started on oral diuretic, beta-blocker with plans to start angiotensin-converting enzyme (ACE) inhibitor at follow-up.
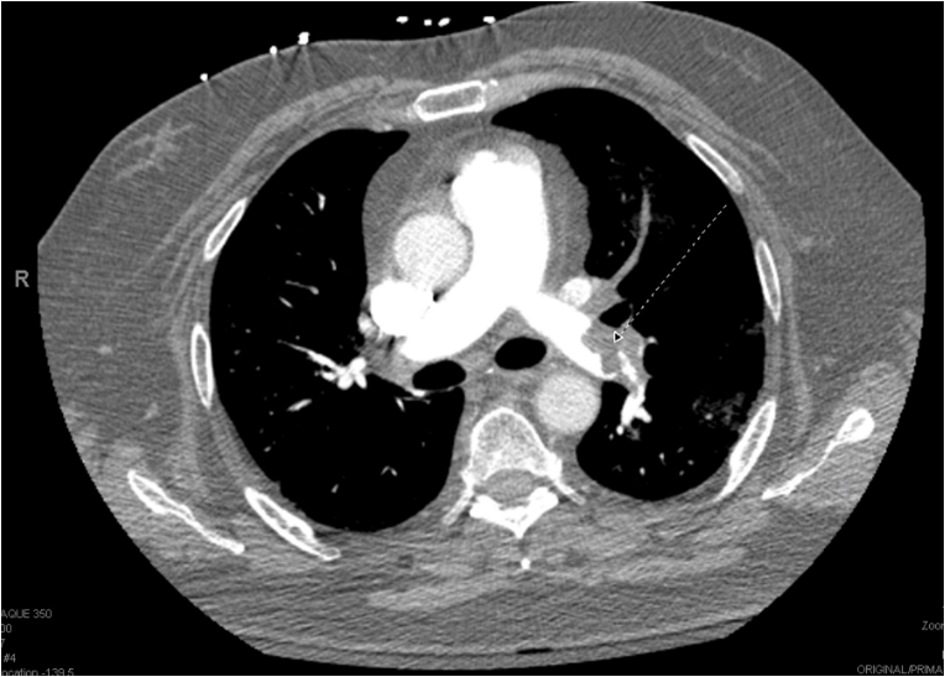 Click for large image | Figure 1. CT angiography of the chest. |
Four days after discharge, patient presented to the emergency department with syncope. Patient sat on the toilet to defecate and called his wife for help as he felt like he was going to pass out. He subsequently lost consciousness for a moment. In the emergency room, patient was hypoxic requiring 2 L of oxygen, tachypnic, and tachycardic (EKG with sinus tachycardia), and normotensive. Due to this episode of syncope that was felt to be secondary to huge clot burden, patient underwent bilateral pulmonary endarterectomy with thrombo-embolectomy (Fig. 2) utilizing deep hypothermic circulatory arrest. Pathological specimen of both right and left pulmonary arteries demonstrated organizing thrombus with admixed carcinoma (Fig. 3). The tumor was strongly positive for cytokeratin AE1/AE3 (Fig. 4) and cytokeratin 7 (CK7), patchy positive for CK20, negative for RCC and PAX2 with histomorphology reminiscent of transitional cell (urothelial carcinoma).
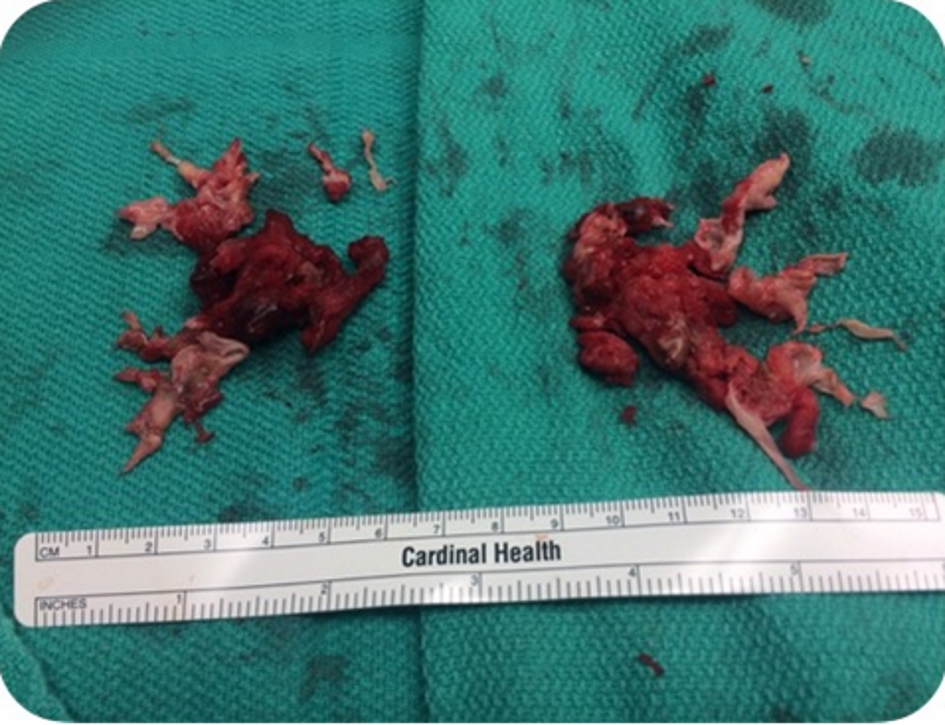 Click for large image | Figure 2. Thrombo-embolectomy specimen. |
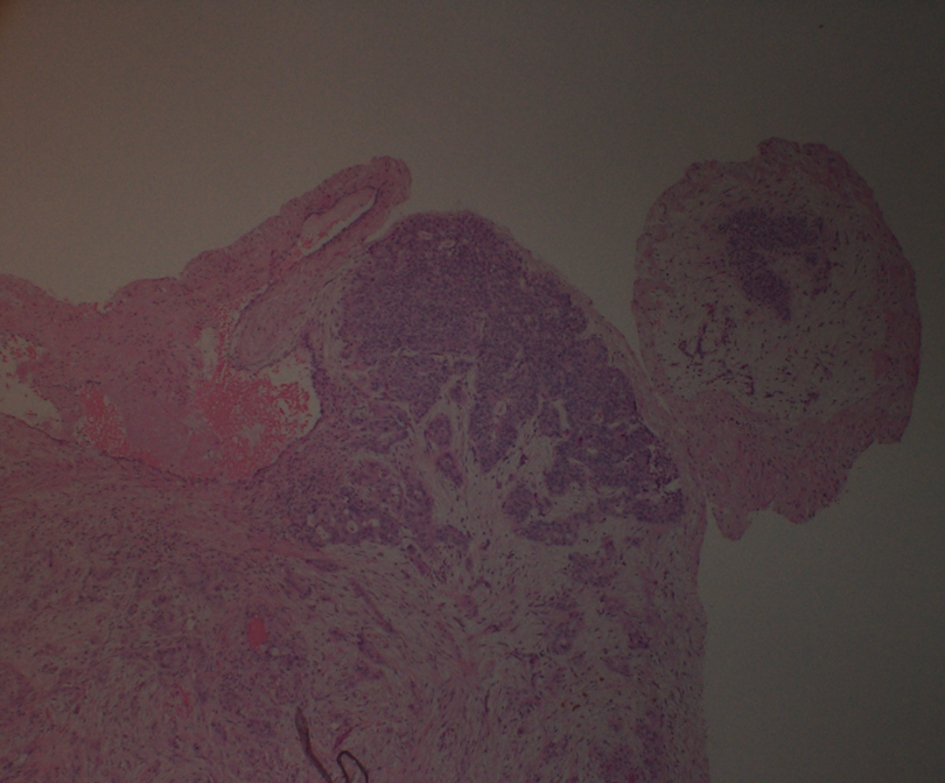 Click for large image | Figure 3. Histopathology of thrombo-embolectomy specimen. |
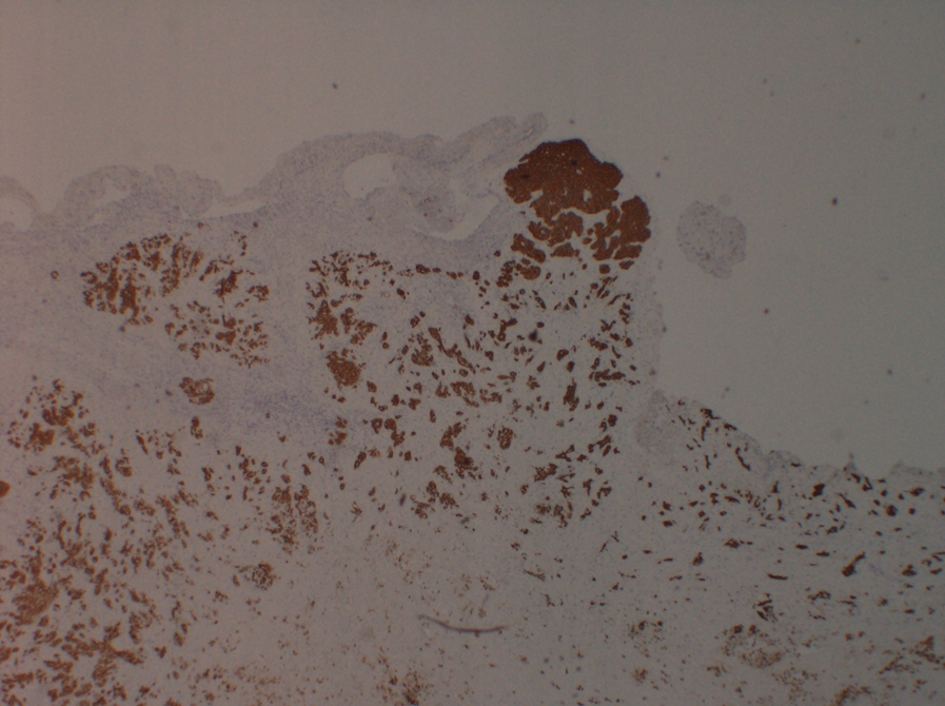 Click for large image | Figure 4. Tumor thrombus staining positive for cytokeratin AE1/AE3. |
With the diagnosis of metastatic urothelial carcinoma in the setting of significant cardiac dysfunction and overall deconditioning, patient was started on palliative chemotherapy with weekly gemcitabine along with lifelong anticoagulation with lovenox. Patient had good functional and clinical recovery from the respiratory standpoint after thrombo-embolectomy but his clinical course was then complicated by acute gastrointestinal (GI) bleeding in the setting of systemic anticoagulation, further metastasis to lumbar spine requiring palliative radiation and pembrolizumab. Due to poor functional status, patient was subsequently transitioned to hospice and expired 7 months after the thrombo-embolectomy.
| Discussion | ▴Top |
Tumor thrombus is a rare complication of solid cancers, mainly renal cell carcinoma, Wilm’s tumor, testicular carcinoma, adrenal cortical carcinoma and hepatocellular carcinoma. Little is known about tumor thrombus to the pulmonary artery from upper urinary tract urothelial carcinoma. In our patient, it is possible that the surgery might have triggered the hematogenous spread given the peri-vascular invasion. While we do not have definite diagnostic criteria to rule out tumor thrombus, it should be in our differential especially in a cancer patient who has failed at least two classes of anticoagulation or progressed in spite of being complaint and/or therapeutic on systemic anticoagulation therapy.
Pulmonary embolus has become more of a clinical diagnosis with infection (septic emboli), blood clot (venous emboli), autoimmunity (heparin-induced thrombocytopenia), and malignancy (tumor emboli) being the different etiologies. It is vital to delve into the etiological reasons for targeted therapy and also to avoid unnecessary anticoagulation. In our patient, anticoagulation was continued, as the pathological diagnosis showed a mixture of thrombus and carcinoma in the pulmonary artery but in typical tumor thrombus, anticoagulation is not warranted.
In our patient, early suspicion might have triggered further non-invasive diagnostic considerations and early initiation of therapy targeting the metastatic cancer.
Rare case reports [1-3] have described 18F-2-deoxy-2-fluoro-D-glucose (FDG) uptake in the lungs (e.g. during serial positron emission tomography (PET) surveillance) when presumably tumor deposits are large enough to be FDG-avid. There are also case reports [4-6] describing the identification of filling defects in the major pulmonary arteries using endobronchial ultrasound (EBUS). Our patient underwent invasive surgical biopsy, which has shown to have the highest diagnostic yield but also has the highest procedural risk.
The optimal management of pulmonary artery tumor thrombus remains unresolved because of the rarity, heterogeneity of the disease, and also due to late/under diagnosis. Traditionally, definitive therapy for tumor thrombus is directed at treating the primary tumor. This may include surgical resection, systemic chemotherapy or a combination of chemo-radiation. Anticoagulation is not routinely administered for tumor thrombus, but in our patient, anticoagulation was continued due to pathological evidence of thrombus in addition to the tumor.
Tumor thrombus is typically an end-stage manifestation of malignancy. With a disease that has high mortality, it is vital to have a high level of suspicion. High index of suspicion would facilitate early diagnosis and early initiation of targeted cancer therapy.
| References | ▴Top |
- Shin S, Pak K, Kim SJ, Kim H, Kim SJ. Pulmonary tumor embolism derived from stomach cancer observation with serial 18F-FDG PET/CT. Clin Nucl Med. 2015;40(3):270-272.
doi pubmed - Sone T, Yoshikawa K, Fukunaga M. Pulmonary tumor embolism from choriocarcinoma: detection with F-18 FDG positron emission tomography. Clin Nucl Med. 2008;33(11):773-774.
doi pubmed - Purandare NC, Dua SG, Rangarajan V, Shah S, Sharma AR. Pulmonary artery and femoral vein tumour thromboembolism in a patient with osteogenic sarcoma demonstrated by FDG PET/CT. Eur J Nucl Med Mol Imaging. 2010;37(3):653.
doi pubmed - Desai NR, Greenhill SR, Vance M, Kovitz KL. Pulmonary tumor embolism diagnosed by endobronchial ultrasound. J Bronchology Interv Pulmonol. 2015;22(4):e16-19.
doi pubmed - Blanc AL, Jardin C, Faivre JB, Le Rouzic O, Do Cao C, Benhamed L, Ramon PP, et al. Pulmonary artery tumour-embolism diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Eur Respir J. 2011;38(2):477-479.
doi pubmed - Chamorro N, Blanco I, Sanchez M, Ramirez J, Barbera JA, Agusti C. The expanding horizons of endobronchial ultrasound: diagnosis of a tumor embolism. Chest. 2012;142(5):1334-1336.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


