| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 2, June 2019, pages 60-63
Durable Response to Venetoclax Monotherapy in Richter’s Syndrome: A Case Report and Review of Literature
Revathi Kolliparaa, d, Kelly Szymanskib, Brett Mahonc, Parameswaran Venugopalb
aDepartment of Internal Medicine, Rush University Medical Center, Chicago, IL, USA
bDivision of Hematology, Oncology and Cell Therapy, Rush University Medical Center, Chicago, IL, USA
cDepartment of Pathology, Rush University Medical Center, Chicago, IL, USA
dCorresponding Author: Revathi Kollipara, Department of Internal Medicine, Rush University Medical Center, 1717 W. Congress Parkway, 1042 Kellogg, Chicago, IL 606012, USA
Manuscript submitted December 21, 2018, accepted February 15, 2019
Short title: Response to Venetoclax Monotherapy in RS
doi: https://doi.org/10.14740/jh473
| Abstract | ▴Top |
In 2017, 20,110 people in the United States were diagnosed with chronic lymphocytic leukemia (CLL). Of these patients, 5-15% will ultimately undergo Richter’s syndrome (RS), a transformation to a more aggressive lymphoma, most commonly diffuse large B-cell lymphoma (DLBCL) type. Particularly when the transformation is clonally related, prognosis is poor in these individuals with a median survival of only 5 - 14 months. This is an area of unmet need, and as such, the benefits of novel approaches with targeted therapies should be explored. Our patient is a 70-year-old female who was diagnosed with CLL in 2010. In 2016, she presented to her general practitioner with new B symptoms and leukocytosis. Cytogenetics on peripheral blood was notable for known trisomy 12 (52.8% of cells) and new 17p deletion (93.4% of cells). She received five cycles of ofatumumab with complete resolution of systemic symptoms but mixed response on interim computed tomography (CT) scan with ensuing rise in her white blood cell (WBC) and lactic acid dehydrogenase (LDH). A positron emission tomography (PET) scan had disproportionate uptake in the porta hepatis lymph nodes and subsequent lymph node biopsy confirmed transformation. She was started on R-CHOP chemotherapy but tolerated it very poorly. She was transitioned to venetoclax monotherapy in April 2017 and achieved a partial response by CT and bone marrow biopsy. This has been maintained over the last 12 months allowing the patient to travel and maintain a high quality of life. While the pathogenesis to RS is poorly understood, there have been several studies to identify tumor genetic changes predisposing to transformation. Of the proposed factors, a review of the literature consistently suggests p53 tumor suppressor gene mutation and/or 17p deletion to be associated with RS. Venetoclax is a selective BCL-2 inhibitor that is now approved for CLL patients with 17p deletion. This case serves as an example encouraging the use and study of novel agents such as venetoclax alone or in combination with traditional regimens or other novel agents to mitigate the poor prognosis of 17p deletion associated RS. Further research, however, is required to clarify the pathogenesis of RS and identify optimal treatment strategies.
Keywords: Chronic lymphocytic leukemia; Richter transformation; Venetoclax
| Introduction | ▴Top |
In 2017, 20,110 people in the United States were diagnosed with chronic lymphocytic leukemia (CLL) [1], and of these patients, 5-15% ultimately undergo Richter’s syndrome (RS) [2, 3] at a transformation rate of about 0.5% per year [4]. RS is the development of high-grade non-Hodgkin’s lymphoma (NHL), with diffuse large B-cell lymphoma (DLBCL) being the predominant type of RS, occurring in up to 90% of all cases [5]. While the pathogenesis to RS is ultimately poorly understood, there have been several studies to identify predisposing risk factors and/or tumor genetic predisposition to transformation. Multiple features are associated with higher risk of RS including polymorphisms in BCL-2 and CD38, advanced Rai stage > stage III at CLL diagnosis, biologic characteristics of the CLL B cell, and the type of chemotherapy used to treat the CLL [4]. Particularly when the transformation is clonally related, prognosis is poor in these individuals with a median survival of only 5 - 14 months with poor depth and duration of response to standard therapeutic options [4, 6]. Currently, national guidelines suggest treatment regimens traditionally designed for de novo DLBCL with consideration for allogeneic stem cell transplantation for appropriate transplant candidates with chemotherapy sensitive disease. However, since the median age at diagnosis of CLL is 70 years [1], many patients are in fact not considered appropriate candidates for allogeneic stem cell transplantation. As such, consideration of novel approaches with targeted therapies that can induce durable remission is an attractive option.
| Case Report | ▴Top |
Our case describes a 70-year-old female with a history of small lymphocytic lymphoma (SLL)/CLL diagnosed from a biopsy of the left supraclavicular node in October 2010. Bone marrow biopsy revealed 75-80% infiltration with CLL/SLL with fluorescence in situ hybridization (FISH) analysis 40% positive for trisomy 12. Imaging showed involvement in the neck, axilla, chest, abdomen, and groin. She completed six cycles of bendamustine and rituximab by April 2011, and achieved complete remission.
In June 2016, she presented to her primary care physician (PCP) with B symptoms and was found to have significant increase in leukocytosis (peak white blood cell (WBC) count was 162,350 and peak absolute lymphocyte count (ALC) was 139,960). FISH studies on peripheral blood was notable for known trisomy 12 (52.8% of cells) and new 17p deletion (93.4% of cells). Imaging revealed progression of the disease; and patient then received five cycles of ofatumumab with complete resolution of systemic symptoms but mixed response on interim computed tomography (CT) scan. WBC and lactic acid dehydrogenase (LDH) continued to rise, so a bone marrow biopsy was done which showed persistent chronic lymphocytic leukemia involving 30% of the marrow space and decreased myelopoiesis with Richter’s transformation evident in the peripheral blood (Fig. 1). A positron emission tomography (PET) scan had disproportionate fluorodeoxyglucose (FDG) uptake in the porta hepatis lymph nodes and subsequent lymph node biopsy confirmed transformation to DLBCL.
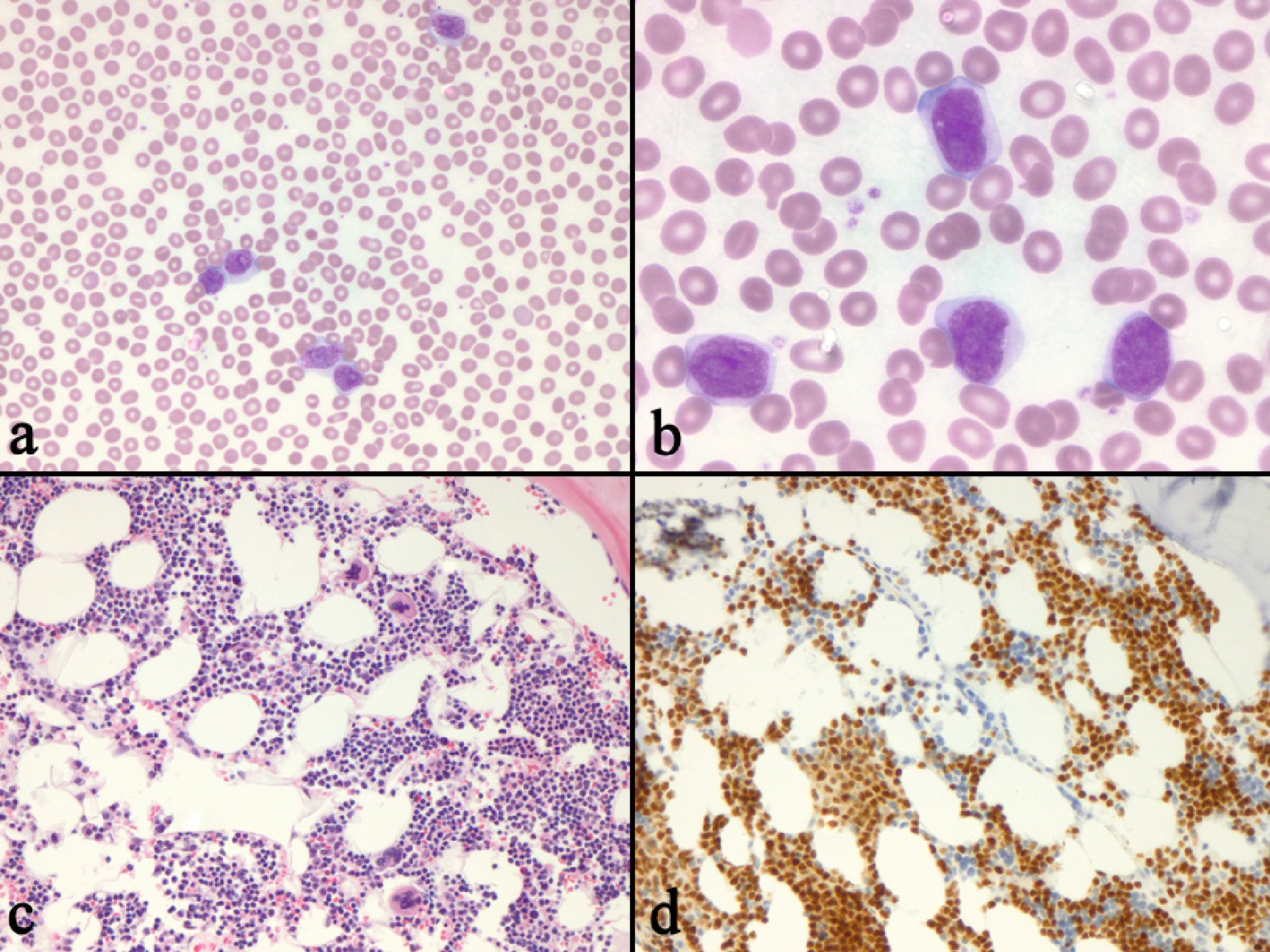 Click for large image | Figure 1. Bone marrow biopsy revealing persistent CLL with transformation to DLBCL. (a) Peripheral blood cells contained “blastoid” appear to be large lymphocytes with moderate amounts of cytoplasm and irregular nuclei (× 200). (b) The cells have large, irregularly shaped nuclei with fine nuclear chromatin, and prominent nucleoli (× 400. (c) The bone marrow biopsy shows an interstitial infiltrate of small to medium sized lymphocytes (× 200. (d) PAX-5 immunostain of the marrow biopsy highlights the interstitial infiltrate (× 200). CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B-cell lymphoma. |
In March 2017, she was started on the first round of R-CHOP chemotherapy, which was complicated by multiple hospital admissions with fevers and confusion. CT scan following the first cycle showed mixed response. Due to intolerance of the chemoimmunotherapy, she was started on venetoclax in April 2017. The patient achieved partial response by CT, which has been maintained since then allowing the patient to maintain a high quality of life.
| Discussion | ▴Top |
With the advent of novel targeted agents, better understanding of the biology and molecular pathways leading to a disease state is essential for selection of optimal therapy as well as accurate prediction of outcome. While the pathogenesis to RS is poorly understood, there have been several studies to identify tumor genetic changes predisposing to transformation. Chigrinova et al [2] studied CLL patient samples of patients with a previous history of CLL, patients in the CLL phase of RS, patients that have undergone RS to DLBCL, patients without transformation, and patients with de novo DLBCL. They found that half of the RS cases (30/60) involved TP53 inactivation and/or CDKN2A loss along with MYC activation. The next most common group occurring in a third of the cases, was those with presence of trisomy 12 [2], which is followed by the acquisition of NOTCH1 mutations. NOTCH1 mutations are mutually exclusive from MYC oncogenic activation supporting the finding that NOTCH1 directly stimulates MYC transcription [3]. Literature showed a more consistent correlation with patients with a p53 tumor mutation and/or 17p deletion and predisposition for RS [2-4, 6, 7].
Venetoclax is BCL-2 specific BH3 mimetic that is now approved for CLL patients with 17p deletion. BH3 mimetics such as navitoclax and venetoclax are agents that help trigger apoptosis by acting as antagonists of BCL-2. High levels of BCL-2 are present in multiple hematologic malignancies including CLL, mantle cell lymphoma, and in up to one-third of DLBCL [8]. Inactivation of TP53 for example can lead to increase in BCL-2 pro-survival proteins or impaired regulation of BH3-only proteins [9], which affects the anti-proliferative effect of chemotherapies [2-4, 6, 7]. The majority of RS-DLBCL patients have a disruption in TP53 so it’s important for targeted therapies to act independently of this gene, which venetoclax is able to do [10].
A phase I trial of venetoclax in relapsed/refractory CLL showed promising results with an overall response rate of 79% and complete remission in 20% of the patients [8]. Further studies are now being done to investigate venetoclax use in other hematologic malignancies. Recently, a phase I trial with venetoclax was recently done in 106 patients with relapsed or refractory non-Hodgkin’s lymphoma. Only three patients had laboratory evidence of tumor lysis syndrome (TLS), but all had a high tumor burden to begin with and in general, the drug was well tolerated. Out of the 106 patients, seven patients had RS/DLBCL and three out of seven of those patients had an overall response but none achieved complete response [11].
Trials are underway studying venetoclax with other chemotherapy and targeted therapeutic agents. One trial published in 2017 that studied ibrutinib and venetoclax in relapsed, refractory CLL patients suggests possible synergy between the two chemo-immunotherapy agents [12]. Clinical trials exploring chemo-immunotherapy-venetoclax are already underway (NCT03054896) as well. Further research, however, is required to clarify the pathogenesis of RS and identify optimal treatment strategies. This case serves as an example encouraging the use and study of novel agents such as venetoclax alone or in combination with traditional regimens or other novel agents to mitigate the poor prognosis of 17p deletion associated RS. Studies using combination of venetoclax with other novel agents are also looking at the possibility of defined duration of therapy in patients who achieve deep remission. This may also apply to patients with RS.
Acknowledgments
We thank the patient for allowing us to share the details of her case.
Financial Disclosure
No disclosure. Publication fee was paid by the Division of Hematology, Oncology and Cell Therapy at Rush University Medical Center.
Conflict of Interest
Parmeswaran Venugopal is a consultant for Bayer and AbbVie pharmaceuticals. Brett Mahon is the director of Pathology for Tempus Laboratories and has equity interest. He is a consultant for AbbVie. Revathi Kollipara and Kelly Szymanski have no disclosures.
Informed Consent
The patient has provided verbal consent for preparation of this case report.
Author Contributions
Authors Kollipara, Szymanski and Venugopal contributed to the manuscript of the project. Author Mahon provided the images and description of Figure 1.
| References | ▴Top |
- SEER. Cancer stat facts: Leukemia - CLL. 2018. Retrieved https://seer.cancer.gov/statfacts/html/clyl.html.
- Chigrinova E, Rinaldi A, Kwee I, Rossi D, Rancoita PM, Strefford JC, Oscier D, et al. Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood. 2013;122(15):2673-2682.
doi pubmed - Fabbri G, Khiabanian H, Holmes AB, Wang J, Messina M, Mullighan CG, Pasqualucci L, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210(11):2273-2288.
doi pubmed - Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123(11):1647-1657.
doi pubmed - Khan M, Siddiqi R, Thompson PA. Approach to Richter transformation of chronic lymphocytic leukemia in the era of novel therapies. Ann Hematol. 2018;97(1):1-15.
doi pubmed - Tsimberidou AM, O'Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, Wen S, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343-2351.
doi pubmed - Rossi D, Cerri M, Capello D, Deambrogi C, Rossi FM, Zucchetto A, De Paoli L, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008;142(2):202-215.
doi pubmed - Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, et al. Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322.
doi pubmed - Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27-36.
doi pubmed - Condoluci A, Rossi D. Treatment of Richter's syndrome. Curr Treat Options Oncol. 2017;18(12):75.
doi pubmed - Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, et al. Phase I First-in-human study of Venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833.
doi pubmed - Hillmen P, et al. Initial results of bloodwise TAP CLARITY study. Session presented at ASH 59th Annual Meeting and Exposition. 2017. Retrieved https://ash.confex.com/ash/2017/webprogram/Paper104020.html.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


