| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 1, March 2019, pages 40-43
Disseminated Intravascular Coagulopathy Secondary to Unintentional Brodifacoum Poisoning via Synthetic Marijuana
Abigail Chana, c, Michael Adasheka, Julian Kanga, Adriana Medinab
aDepartment of Internal Medicine, Sinai Hospital of Baltimore, Baltimore, MD, USA
bDepartment of Medical Oncology, Sinai Hospital of Baltimore, Baltimore, MD, USA
cCorresponding Author: Abigail Chan, Department of Internal Medicine, Sinai Hospital of Baltimore, 2401 W Belvedere Ave., Baltimore, MD 21210, USA
Manuscript submitted February 16, 2019, accepted March 20, 2019
Short title: DIC via Synthetic Marijuana
doi: https://doi.org/10.14740/jh486
| Abstract | ▴Top |
Recent evidence demonstrates a rising epidemic of unintentional brodifacoum poisoning associated with synthetic cannabinoid use. Synthetic cannabinoid use is on the rise because of its inexpensiveness as well as difficulty to screen and regulate. We present a rare case of severe coagulopathy and cardiac arrest secondary to synthetic cannabinoid use complicated by brodifacoum toxicity.
Keywords: Disseminated intravascular coagulopathy; Synthetic marijuana; Brodifacoum poisoning
| Introduction | ▴Top |
Synthetic cannabinoids (SCs) are inexpensive and have quickly spread worldwide since introduction in the early 21st century. The multiple analogs of SCs render urine drug testing difficult [1] and routine urine toxicology ineffective [2]. This subsequent explosion in SC use has coincided with an alarming increase in complications including myocardial infarction [2-6], cerebrovascular disease [7, 8], psychosis [9], seizures [10], acute kidney failure [11-14], and death [3, 15]. We present a rare case of severe coagulopathy and cardiac arrest secondary to SC and unintentional brodifacoum exposure.
| Case Report | ▴Top |
A 38-year-old man with previous diagnoses of bipolar disorder, post-traumatic stress disorder and polysubstance abuse presented with a 3-day history of epistaxis, hematuria, rectal bleeding, bruising and diffuse abdominal pain. His medical history was positive for daily marijuana as well as synthetic marijuana use and was otherwise negative. Vital signs were normal. His physical exam was significant for ecchymosis of the abdomen and extremities.
Initial workup was remarkable for elevated prothrombin time (PT) at > 154 s (normal range (NR): 9.2 - 11.8 s) and activated partial thromboplastin time (aPTT) at > 169 s (NR: 23 - 30 s), as well as an undetectably high international normalized ratio (INR) (NR: 0.9 - 1.1 s). D-dimer was reported to be > 35 mg/L (NR: 0.19 - 0.9 mg/L), lactate dehydrogenase (LDH) was elevated at 582 Unit/L (NR: 84 - 246 Unit/L) and low fibrinogen was 64 mg/dL (214 - 407 mg/dL) without schistocytes on peripheral blood smear. Liver function tests were within normal limits. His complete blood count showed white blood cell count (WBC) of 9,870/mm3, hemoglobin of 15.9 g/dL, hematocrit of 47.2%, and platelets of 287,000/mm3. Urine toxicology was positive for both cannabinoids and opioids, and urinalysis was consistent with hematuria.
The patient was diagnosed with disseminated intravascular coagulopathy (DIC). Initial resuscitative efforts included intravenous fluids, fresh frozen plasma, cryoprecipitate, vitamin K1, and factor IX concentrate. The patient was found hours later outside his hospital room unconscious and in pulseless electrical activity (PEA) arrest. The patient did have return of spontaneous circulation but the etiology of his cardiac arrest remained unknown. Maryland Poison Control was contacted, who were concerned for potential brodifacoum-laced synthetic cannabinoid use. Over the course of his hospital stay, he required immense doses of vitamin K1, in oral and intravenous doses. His INR trends and treatment measures are depicted in Figure 1.
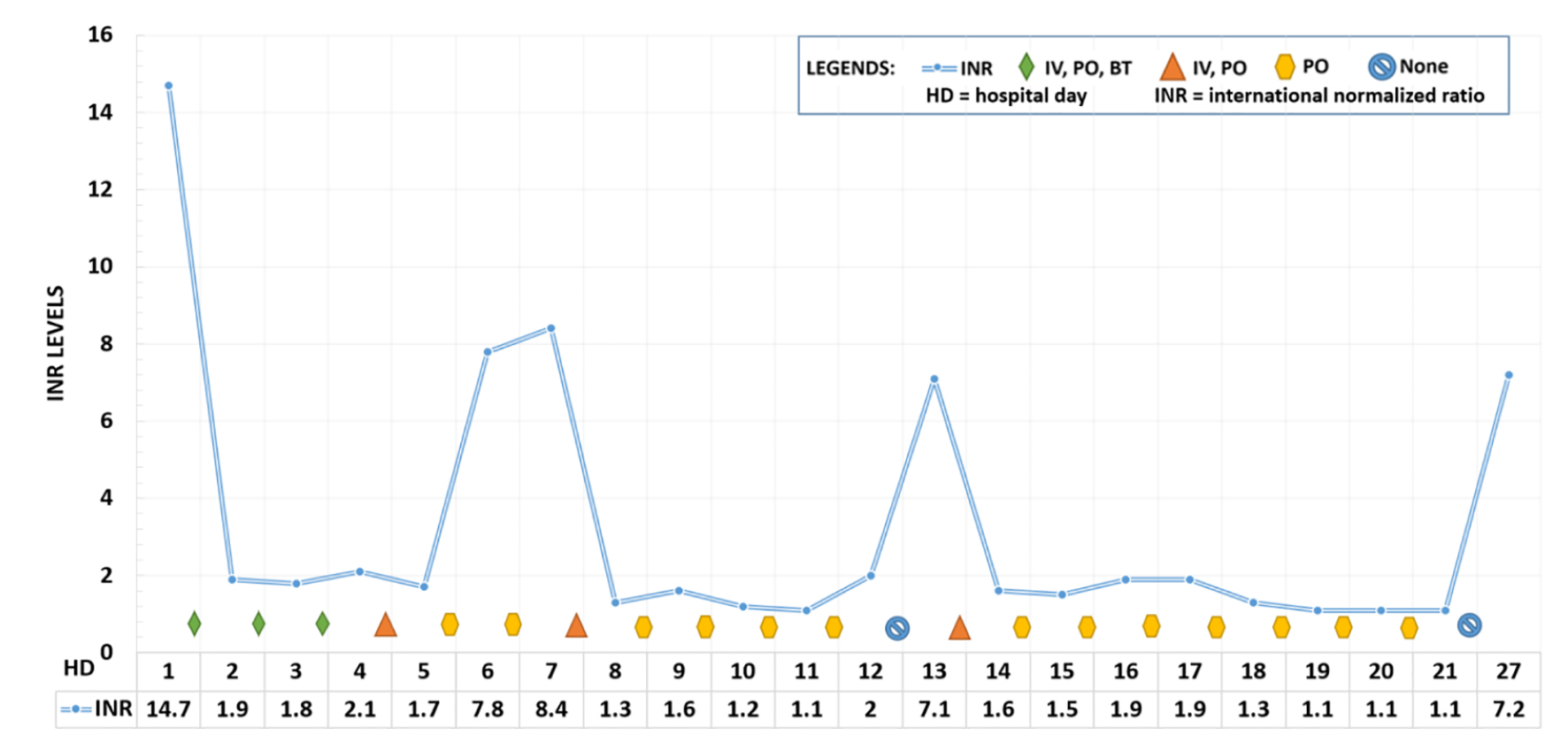 Click for large image | Figure 1. Daily INR level and treatment regimen while hospitalized. INR on admission was extrapolated from aPPT levels. Initial treatment utilized intravenous and oral vitamin K, reduced to oral vitamin K supplementation alone on day 5, with intermittent infusions. He refused the dose on day 12. Patient was discharged on day 21. He returned to the emergency room on day 27. BT: blood transfusion; IV: intravenous vitamin K1; PO: oral vitamin K1; HD: hospital day; INR: international normalized ratio. |
The patient was successfully discharged on daily oral vitamin K with a discharge INR of 1.1. Unfortunately, the patient was noncompliant with his medication and presented 3 days after discharge to the emergency department with new onset left upper extremity deep vein thrombosis and an INR of 7.2. He was given intravenous vitamin K in the emergency department but left against medical advice and was lost to follow up.
| Discussion | ▴Top |
SC toxicity is on the rise in the United States of America (USA) [16]. SCs come in many varieties, and clinicians should be aware of the current monikers including “Spice”, “K2”, “Cloud 9” among many others [17]. Common symptoms of SC exposure consist of agitation, depression, psychosis and coma [16] as well as tachycardia, hypertension, chest pain, hallucinations and vertigo [17]. A recent case series demonstrated that out of 456 patients treated for SC intoxication, 277 reported SC as the sole toxic agent. Among these cases, >25% occurred in the pediatric population between ages 13 and 18 years old, and 83.1% of patients were male [16]. Caviness et al reported that SC use often coincides with binge drinking and recreational drug use including marijuana [18]. No antidotes to the effects of SCs currently exist [16].
From March to July of 2018, the Illinois Department of Health reported 255 patients with SC-associated coagulopathy, and eight mortalities [19]. Kelkar and colleagues identified 34 patients with SC-associated coagulopathy with a mean age of 37 years old, and presenting symptoms of gross hematuria and abdominal pain. Mean INR on presentation was 15.8. Vitamin K1 (phytonadione) was given orally to all patients and 68% of cases were supplemented with intravenous vitamin K1, 55% with fresh frozen plasma, and one case with 4-factor prothrombin complex concentrate. Eight patients left against medical advice and six were subsequently readmitted. Serum samples from these patients tested positive for brodifacoum [20]. As of January 1, 2019, the Maryland Poison Center at the University of Maryland School of Pharmacy reported notification of 44 cases with exposure to SC with significant elevations in INR and hemorrhage with 9% mortality [21].
In the USA, synthetic cannabis production remains illegal in federal law, and therefore federally unregulated [22]. At this time, it is unknown how brodifacoum was incorporated into the patient’s SC; however, in cannabis production facilities, brodifacoum is often applied to the base of Cannabaceae stalks as a rodenticide. Quantities up to 25 kg can be found at these facilities, and have been correlated to an increased death toll on local animals [23]. Compared to warfarin, the strong hydrophobicity in brodifacoum allows for longer tissue retention, a half-life lasting from 20 days to 12 months, and a potency 100 times stronger than warfarin in reducing vitamin K-dependent coagulation factors [24]. Toxicity in rat models was higher when inhaled than ingested [25]. Unfortunately, diagnosis remains difficult. A recent study by Ng et al stated that in 41 identified cases of extended release warfarin toxicity, 25% of patients did not have obvious exposure history, nor could identify the causative agent. Occult poisoning was frequently missed on initial clinician visit, leading to delayed initiation of treatment [26].
The Saint Frances Medical Center in Illinois has developed a criterion to diagnose SC-associated coagulopathy. Major criteria include: 1) presence of vitamin K-dependent factor coagulopathy (defined as a prothrombin time ≥ 14.8 s and an INR ≥ 1.3); and 2) recent exposure to SCs (within the past 30 days). The minor criteria include: 1) active bleeding symptoms; 2) exposure to contaminated SCs obtained from a person with known superwarfarin poisoning; and 3) positive toxicology for superwarfarin. The use of prescribed anticoagulants was listed as a confounding factor. Patients with both major criteria and at least one minor criterion were diagnosed with SC-associated coagulopathy. In cases of concurrent anticoagulant use, an anticoagulant poisoning panel, which detects warfarin, dicumarol, diphacinone, chlorophacinone, difenacoum, brodifacoum, and bromadiolone, was utilized [20].
Treatment of brodifacoum toxicity depends greatly on the method of poisoning [27]. Studies in a canine animal model have demonstrated that if emesis is induced within 1 h of ingesting brodifacoum rodenticide, 10-77% of brodifacoum is expelled with the gastric contents. In this canine population, all the animals did well without further medical treatment and did not require further medical intervention [28]; however, this has not been studied in a human model. In humans, the rapid correction of severe coagulopathy can be achieved with a combination of the following interventions: fresh frozen plasma, recombinant activated factor VII, prothrombin complex concentrate, intravenous and oral vitamin K1 [24].
In all cases of brodifacoum toxicity with elevated PT, vitamin K1 should be administered via slow intravenous injection of 10 - 25 mg every 3 - 6 h until PT has normalized. Subsequently the patient should be prescribed 10 mg of oral vitamin K1 four times a day [27]. Extensive follow-up and monitoring for months will be required due to brodifacoum’s half-life with eventual taper of oral vitamin K1 [24, 27]. The financial burden on the patient and the healthcare system is a great one, as a 1-month supply of vitamin K1 costs between $24,000 and $34,000 (US dollars) [20].
In conclusion, there is evidence of a rising epidemic of brodifacoum poisoning as a result of SC use. There may be some evidence that brodifacoum is used as a rodenticide to maximize crop production from illegal synthetic cannabis producing facilities, and the toxicities are passed along to uninformed consumers. If acutely ingested, emesis is a viable initial option as front-line treatment, while results of PT and aPTT are pending. Supportive measures and treatment with vitamin K1 should be initiated once the coagulability is identified and continued on discharge. Long-term administration of vitamin K1, frequent laboratory monitoring, and close follow-up with medical providers comes at a high cost and an interdisciplinary team consisting of medical providers, pharmacists and social workers are warranted. Synthetic cannabis use in the pediatric population is especially concerning, and pediatricians as well should be vigilant for signs of hematuria, ecchymosis, abdominal pain or rectal bleeding as all may be an initial sign of brodifacoum toxicity. This is an impending public health crisis that many providers may face, and through both public awareness and health education can brodifacoum toxicity be addressed.
Acknowledgments
None.
Financial Disclosure
None of the authors have any financial or personal bias to declare.
Conflict of Interest
None.
Informed Consent
Manuscript has been anonymized and no identifiable information has been included in the manuscript.
Author Contributions
AC, MA, JK and AM equally contributed in the writing of the paper. All authors edited and approved the final manuscript.
Abbreviations
aPTT: activated partial thromboplastin time; BT: blood transfusion; DIC: disseminated intravascular coagulopathy; HD: hospital day; INR: international normalized ratio; IV: intravenous; LDH: lactate dehydrogenase; NR: normal range; PEA: pulseless electrical activity; PO: oral; PT: prothrombin time; SC: synthetic cannabinoid; WBC: white blood cell count; USA: United States of America
| References | ▴Top |
- Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12-41.
doi pubmed - Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128(6):e1622-1627.
doi pubmed - McKeever RG, Vearrier D, Jacobs D, LaSala G, Okaneku J, Greenberg MI. K2 - not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: a case report. J Med Toxicol. 2015;11(1):129-131.
doi pubmed - Atik SU, Dedeoglu R, Varol F, Cam H, Eroglu AG, Saltik L. Cardiovascular side effects related with use of synthetic cannabinoids "bonzai": two case reports. Turk Pediatri Ars. 2015;50(1):61-64.
doi pubmed - Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103(23):2805-2809.
doi pubmed - Daccarett M, Freih M, Machado C. Acute cannabis intoxication mimicking brugada-like ST segment abnormalities. Int J Cardiol. 2007;119(2):235-236.
doi pubmed - Moussouttas M. Cannabis use and cerebrovascular disease. Neurologist. 2004;10(1):47-53.
doi pubmed - Mouzak A, Agathos P, Kerezoudi E, Mantas A, Vourdeli-Yiannakoura E. Transient ischemic attack in heavy cannabis smokers - how 'safe' is it? Eur Neurol. 2000;44(1):42-44.
doi pubmed - Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118(1-3):309-310.
doi pubmed - Babi MA, Robinson CP, Maciel CB. A spicy status: Synthetic cannabinoid (spice) use and new-onset refractory status epilepticus-A case report and review of the literature. SAGE Open Med Case Rep. 2017;5:2050313X17745206.
- Kazory A, Aiyer R. Synthetic marijuana and acute kidney injury: an unforeseen association. Clin Kidney J. 2013;6(3):330-333.
doi pubmed - Zarifi C, Vyas S. Spice-y kidney failure: a case report and systematic review of acute kidney injury attributable to the use of synthetic cannabis. Perm J. 2017;21:16-160.
doi - Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2013;8(4):523-526.
doi pubmed - Srisung W, Jamal F, Prabhakar S. Synthetic cannabinoids and acute kidney injury. Proc (Bayl Univ Med Cent). 2015;28(4):475-477.
doi - Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54(1):1-13.
doi pubmed - Riederer AM, Campleman SL, Carlson RG, Boyer EW, Manini AF, Wax PM, Brent JA, et al. Acute poisonings from synthetic cannabinoids - 50 U.S. toxicology investigators consortium registry sites, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(27):692-695.
doi pubmed - Mills B, Yepes A, Nugent K. Synthetic Cannabinoids. Am J Med Sci. 2015;350(1):59-62.
doi pubmed - Caviness CM, Tzilos G, Anderson BJ, Stein MD. Synthetic cannabinoids: use and predictors in a community sample of young adults. Subst Abus. 2015;36(3):368-373.
doi pubmed - Raja Ali. Bleeding from synthetic cannabinoid additives: coming to an ED near you? NEJM Journal Watch. https://www.jwatch.org/na47534/2018/10/05/bleeding-synthetic-cannabinoid-additives-coming-ed-near. Updated October 5, 2018. Accessed January 12, 2019.
- Kelkar AH, Smith NA, Martial A, Moole H, Tarantino MD, Roberts JC. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379(13):1216-1223.
doi pubmed - Synthetic cannabis update. Maryland Poison Control Center. University of Maryland School of pharmacy. https://www.mdpoison.com/news/2018/synthetic-cannabinoids-updates. Updated January 1, 2019. Accessed January 12, 2019.
- Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69(3):284-288.
doi pubmed - Franklin AB, Carlson PC, Rex A, Rockweit JT, Garza D, Culhane E, Volker SF, et al. Grass is not always greener: rodenticide exposure of a threatened species near marijuana growing operations. BMC Res Notes. 2018;11(1):94.
doi pubmed - Feinstein DL, Akpa BS, Ayee MA, Boullerne AI, Braun D, Brodsky SV, Gidalevitz D, et al. The emerging threat of superwarfarins: history, detection, mechanisms, and countermeasures. Ann N Y Acad Sci. 2016;1374(1):111-122.
doi pubmed - Brodifacoum health and safety guide. Geneva: World Health Organization, International Programme on Chemical Safety, 1995.
- Ng WY, Ching CK, Chong YK, Ng SW, Cheung WL, Mak TWL. Retrospective study of the characteristics of anticoagulant-type rodenticide poisoning in Hong Kong. J Med Toxicol. 2018;14(3):218-228.
doi pubmed - Olmos V, Lopez CM. Brodifacoum poisoning with toxicokinetic data. Clin Toxicol (Phila). 2007;45(5):487-489.
doi pubmed - Parton KH, Willson EK, Collett MG, Booth LH. Recovery of brodifacoum in vomitus following induction of emesis in dogs that had ingested rodenticide bait. N Z Vet J. 2018;66(1):41-43.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


