| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 8, Number 2, June 2019, pages 47-54
Plasma Factors for the Differentiation of Hodgkin’s Lymphoma and Diffused Large B Cell Lymphoma and for Monitoring Remission
Qun Zenga, b, d, Arunima Guptaa, d, Liu Xinc, Michelle Poonc, e, Herbert Schwarza, b, e, f
aDepartment of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
bNUS Immunology Programme, Life Sciences Institute, National University of Singapore, Singapore, Singapore
cDepartment of Haematology-Oncology, National University Health System, Singapore, Singapore
dCo-first authors
eCo-senior authors
fCorresponding Author: Herbert Schwarz, Department of Physiology, National University of Singapore, 2 Medical Dr., Singapore, Singapore
Manuscript submitted May 13, 2019, accepted June 13, 2019
Short title: Plasma Factor Analysis for Hodgkin’s Lymphoma
doi: https://doi.org/10.14740/jh499
| Abstract | ▴Top |
Background: Hodgkin lymphoma (HL) is one of the most frequent cancers occurring at a young age. Although diagnosis of HL is not difficult, a minimally invasive method to diagnose HL, and a radiation-free method to confirm the remission status are highly desired.
Methods: In this study, we employed cutting-edge Luminex technology to evaluate 67 soluble plasma proteins for their suitability for diagnosis and for confirming remission of classical HL (cHL).
Results: Soluble cluster of differentiation (CD)30 and CC motif chemokine ligand (CCL)22 were identified to be capable of differentiating cHL patients from healthy donors and from patients with diffuse large B cell lymphoma (DLBCL), a disease that shares many characteristics with cHL. Soluble tumor necrosis factor receptor (TNFR)2 was found to be lower in the remission than in the initial diagnosis cohort of cHL patients, and also to be lower in plasmas at remission than in plasmas at initial diagnosis from the same patients. In DLBCL plasmas, concentrations of interleukin (IL)-2, soluble IL-2 receptor and IL-31 changed in patients upon entering remission.
Conclusions: Measurement of these factors may: 1) provide a minimally-invasive method to diagnose and differentiate HL and DLBCL, and 2) make it possible to monitor the remission status of these patients without use of radiation-based imaging.
Keywords: CCL22; CD30; Diffuse large B cell lymphoma; Hodgkin lymphoma; IL-2; IL-2R; IL-31; TNFRII
| Introduction | ▴Top |
Hodgkin lymphoma (HL) is one of the most common cancers in children and adolescents in the United States [1], exhibiting a typical bimodal distribution of incidence with those aged 20 to 34 years old being the most frequent patients [2]. HL is classified into two groups: nodular lymphocyte-predominant HL and classical HL (cHL), with cHL making up over 90% of HL cases.
cHL is a cancer of immune cells and is characterized by large Hodgkin and Reed-Sternberg (HRS) cells which are surrounded by a large tumor stroma consisting of non-tumor cells [3, 4]. Lymph nodes are the commonly affected tissues but other organs can also be affected by metastasis [5]. To establish a definitive diagnosis of cHL, HRS cells have to be identified. As HRS cells in the tumor tissue are rare, often constituting less than 1% of the tumor mass, excisional biopsy of the involved lymph nodes rather than fine-needle aspiration cytology or core-needle biopsy is required to provide adequate specimen for pathological identification [6].
Some challenges faced in trying to establish a diagnosis of HL include the difficulties obtaining adequate tissue biopsies in patients with poorly accessible lymph nodes, as well as difficulties in morphological subtyping in a number of cases especially with limited tissue samples. To date, there is no alternative or complementary diagnostic strategy to this highly invasive biopsy method, nor is there a widely adopted non-invasive or minimally invasive screening method for cHL.
The 5-year survival rate is high in patients with Hodgkin lymphoma due to the advent of combined modality (chemotherapy and radiotherapy) treatment [1, 2]. Even in patients with relapsed disease, there are many good salvage regimens with high overall response rates including monoclonal antibody therapy, such as brentuximab vedotin (an anti- cluster of differentiation (CD)30 antibody-drug conjugate) and checkpoint inhibitors [2, 7, 8]. Chimeric antigen receptor T cell therapy is another option [7]. Because of the high cure rates, the focus of cHL treatment has shifted to minimizing the toxicity while preserving the efficacy of the therapy [2]. 18F-fluorodeoxyglucose (FDG) is an isotope-labeled glucose that accumulates in the tumor [9]. Combined with positron emission tomography-computed tomography (PET-CT), FDG PET-CT has achieved great success in the staging of HL and the evaluation of responses to the treatment, allowing for PET-adapted treatment strategies in the management of these cases. However, potential harm caused by radiation is still a concern, especially for the frequent post-treatment evaluation. A minimally-invasive and radiation-free method for confirming response and remission is desired.
Besides HL, the remaining lymphoma are collectively referred as non-Hodgkin lymphoma (NHL), with diffused large B cell lymphoma (DLBCL) being the most common NHL. DLBCL is a heterogeneous entity of different subclasses but share similar morphologies as its name indicates: diffusely grown large B cells [10]. The definitive diagnosis of DLBCL is also based on histological examination of a biopsy of affected lymph nodes. Similarly, FDG PET-CT also provides prognostic values for the treatment of DLBCL [11].
We hypothesized that soluble factors of the plasma could reflect the disease status of cHL. By using Luminex technology and enzyme-linked immunosorbent assay (ELISA), a total of 67 soluble factors (cytokines, soluble receptors, and growth factors) were measured simultaneously and compared among healthy controls, cHL patients and DLBCL patients. Six soluble factors were identified to have potential value for diagnosis and treatment efficacy monitoring.
| Materials and Methods | ▴Top |
Patient plasma
The plasma samples of cHL and DLBCL patients were collected after informed consent during daily clinical practice at the National University Hospital Singapore with approval from the internal review board (NHG DSRB Ref: 2015/00176). Plasma samples of healthy controls were collected with the consent of the donors. All plasma samples were frozen and stored at -80 °C until use. The descriptive statistics of the patients and healthy controls are listed in Table 1. This study was conducted in compliance with the ethical standards of the National University of Singapore on human subjects as well as with the Helsinki Declaration.
 Click to view | Table 1. Statistics of the Patients |
Luminex
The Immune Monitoring 65-Plex Human ProcartaPlex™ Panel (Cat# EPX650-10065-901), the customized 20-plex (stromal cell-derived factor (SDF)-1α, CXC motif chemokine ligand (CXCL)13, interleukin (IL)-1β, IL-2, IL-2R, IL-6, CD137, Eotaxin, CC motif chemokine ligand (CCL)22, IL-31, stem cell factor (SCF), interferon (IFN)-γ, tumor necrosis factor receptor (TNFR)2, a proliferation-inducing ligand (APRIL), monokine induced by gamma interferon (MIG), macrophage inflammatory protein (MIP)-3α, matrix metalloproteinase (MMP)-1, B-cell activating factor (BAFF), macrophage migration inhibitory factor (MIF), and vascular endothelial growth factor (VEGF)A), and the Human ProcartalPlex™ Simplex kits (tumor necrosis factor (TNF)-α, CD30, CD137 ligand (CD137L) and IL-15) were purchased from ThermoFisher Scientific. The 65-plex was run on Bio-Plex® 200 Systems (Bio-Rad) while the 20-plex and Simplex were run on MAGPIX® (Luminex) according to the protocol.
ELISA
CD137 (R&D Systems) and CD137L (LSBio) ELISA were done according to the protocol.
Statistical analysis
Data were analyzed by IBM SPSS Statistics 24 and GraphPad Prism 6. Figures were drawn by GraphPad Prism 6.
| Results | ▴Top |
Patient demographics and responses to treatment
We compared the plasma of cHL patients with plasma of patients with DLBCL and of healthy controls. The first round of analysis (screening set) included 49 cHL patients with 13 new cHL cases, 22 patients initially diagnosed with DLBCL and 12 healthy controls (Table 1). In terms of responses to treatment, 73.5% of the cHL patients and 59.1% DLBCL patients achieved complete remission. The second round of analysis (verification set) included eight pairs of plasma samples from cHL patients and eight pairs of plasma samples from DLBCL patients that were collected before treatment and after remission.
Soluble factors for diagnosing cHL
To explore if and which soluble factors have diagnostic value, plasma of newly diagnosed cHL patients was compared to plasma of healthy controls and plasma of newly diagnosed DLBCL patients. Thirty of the 67 soluble factors were found to be significantly different between the plasma of initially diagnosed cHL patients and of healthy controls (Table 2), indicating a profound disorder in cHL patients’ homeostasis. Compared to DLBCL, a disease that shares many characteristics with cHL, cHL patients showed significant differences in four soluble factors (Table 2). Taking the two comparisons together, we found three shared soluble factors of diagnostic value which were significantly higher in newly diagnosed cHL patient plasma than in healthy control and DLBCL plasma: soluble CD30 (sCD30), CCL22, and CXCL13.
 Click to view | Table 2. Soluble Factors That Occur at Concentrations Significantly Different Between the Plasma of Initially Diagnosed cHL Patients and Healthy Controls |
For sCD30, the median concentration of the cHL patient plasma was 885 pg/mL, and all cHL cases were above the maximum of the healthy controls and the median of the DLBCL cases (Fig. 1a). With a median concentration of 619.6 pg/mL, 84.6% of the cHL patients had CCL22 levels higher than the median level of healthy controls, and 76.9% had a higher level than the median of the DLBCL patients (Fig. 1b). The median CXCL13 concentration in the cHL cases was 279.8 pg/mL (Fig. 1c). All of the CXCL13 levels in cHL plasma were above the median of the healthy controls, and 69.2% of the cHL plasma levels were above the median of the DLBCL cases. These data show that sCD30 is more robust than CCL22 or CXCL13 for the diagnosis of cHL. However, the difference in median cytokine levels between cHL and DLBCL is less prominent for sCD30 and CXCL13 than for CCL22: sCD30 and CXCL13 levels in cHL are only 30% and 60.3%, respectively, higher than the median level in DLBCL. In contrast, CCL22 levels in cHL are 262.8% higher than in DLBCL, suggesting that CCL22 is best suited for differentiating cHL and DLBCL patients. In summary, sCD30 has been found most suited for diagnosing cHL, while CCL22 is best suited for differentiating cHL from DLBCL.
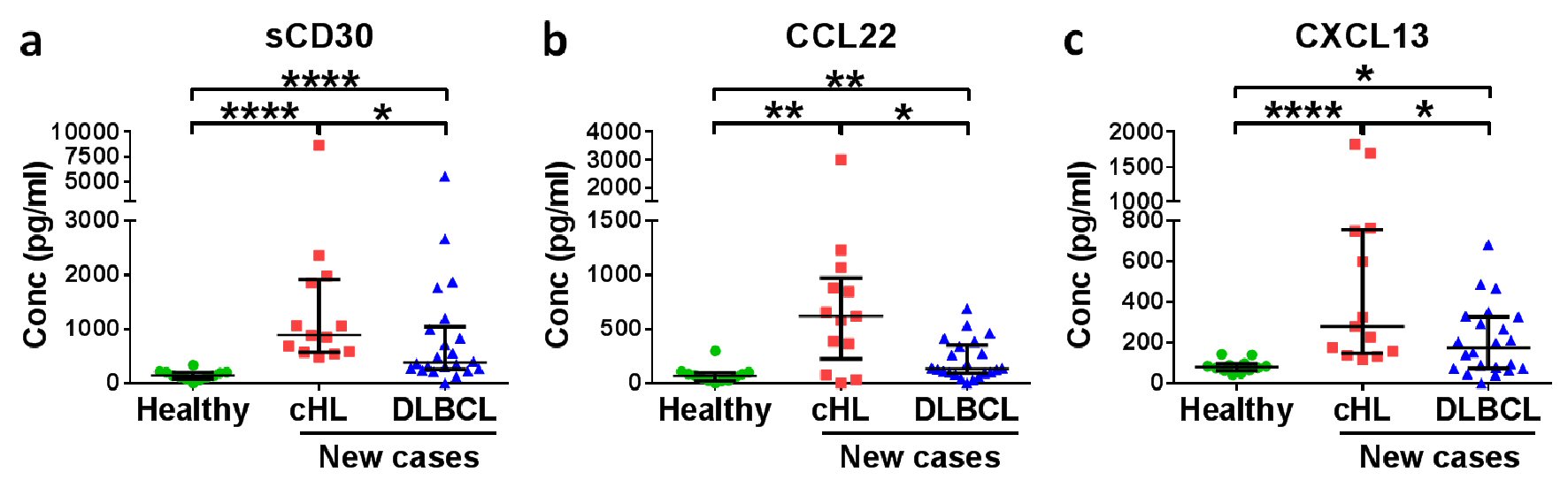 Click for large image | Figure 1. Soluble CD30 (sCD30) and CCL22 have diagnostic potential for HL patients. Medians with interquartile ranges of (a) sCD30, (b) CCL22 and (c) CXCL13 plasma levels in healthy donors, new cases of HL patients and DLBCL patients are shown. *P < 0.05, **P < 0.01, ****P < 0.0001 (Mann-Whitney U test). |
Screening of soluble factors to confirm remission status
To explore if certain soluble factors can be used as a radiation-free alternative for confirming the remission status, we compared the 67 soluble factors in plasma of cHL patients at initial diagnosis and during remission. One soluble factor (eotaxin) was higher (Fig. 2a) and 19 soluble factors were lower (Fig. 2b-s) in cHL patients in remission.
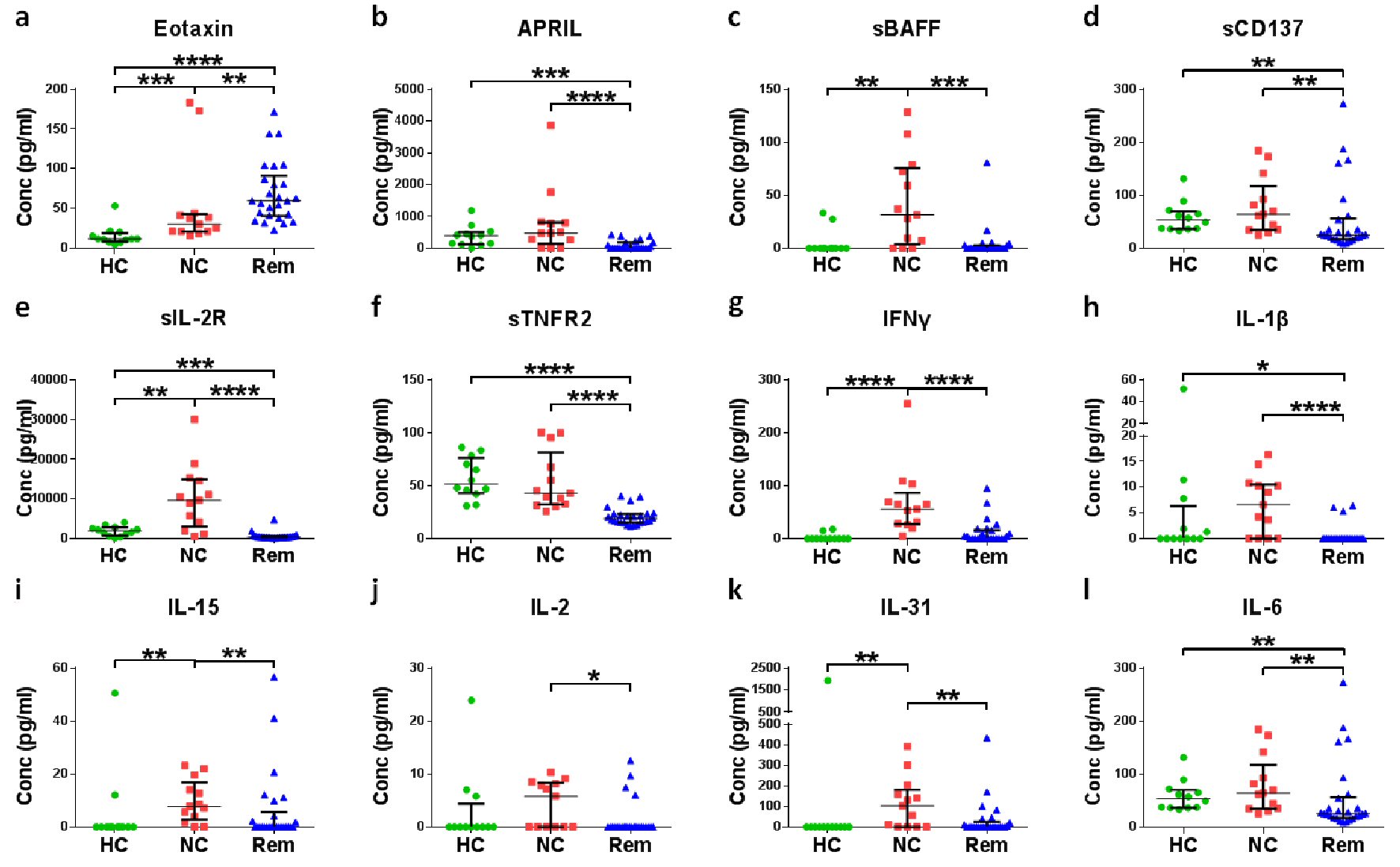 Click for large image | Figure 2. Screening of soluble factors for the confirmation of remission status of cHL. Plasma of different cHL patients at diagnosis (new cases (NC)) were compared with plasma of healthy controls (HC) and plasma of cHL patients in remission (Rem). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Mann-Whitney test). |
Verification of soluble factors to confirm remission status
Since the remission plasmas and new cases plasmas are from different cHL patients, the observed differences may be confounded by donor variation. To validate the remission soluble factors, eight pairs of plasma samples were taken from eight cHL patients. One sample at initial diagnosis and one after the completion of treatment with the patient confirmed to be in remission. These eight pairs were assessed for changes in the above identified factors. Only sTNFR2 levels changed statistically significantly (median: 24.03 vs. 10.83 pg/mL, P < 0.05) between diagnosis and remission (Fig. 3).
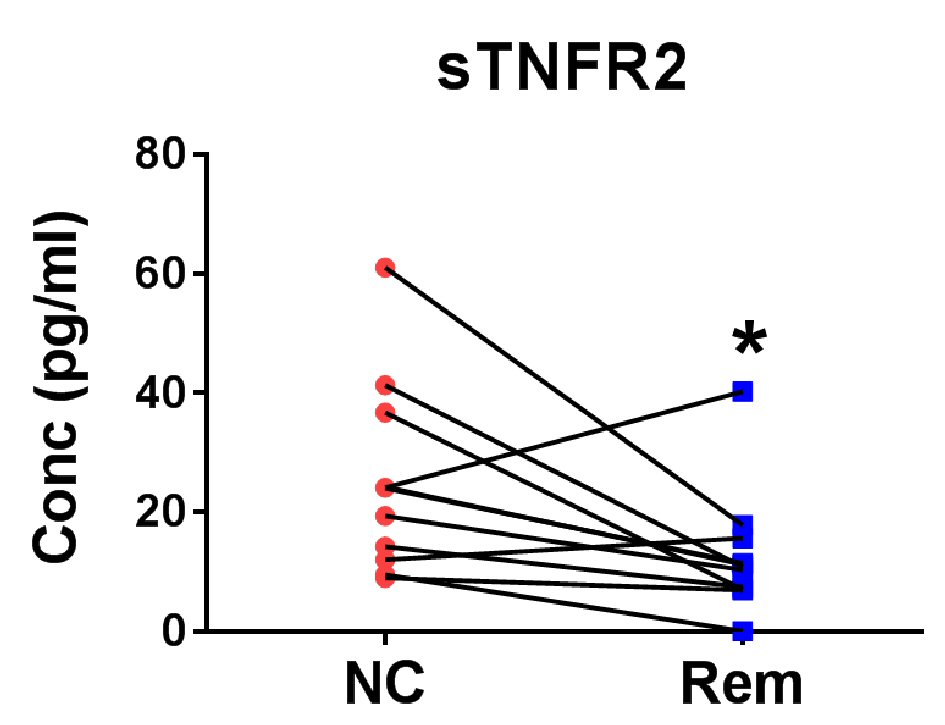 Click for large image | Figure 3. Verification of soluble factors for the confirmation of remission status of cHL. Eight pairs of cHL patient plasma before treatment (new case (NC)) and after remission (Rem) were measured for TNFR2 level. *P < 0.05 (two-tailed, Wilcoxon matched-pairs signed rank test). |
Soluble factors to assess remission in DLBCL
Similarly, eight pairs of plasma samples collected from DLBCL patients at initial diagnosis and in remission were used to screen for soluble factors for the confirmation of remission of DLBCL. IL-2 (median 21.30 vs. 27.92 pg/mL, P < 0.05) (Fig. 4a) was raised in remission whereas sIL-2R (median 1615.0 vs. 503.8 pg/mL, P < 0.05) (Fig. 4b) and IL-31 (median 41.93 vs. 13.32 pg/mL, P < 0.05), (Fig. 4c) were reduced in remission.
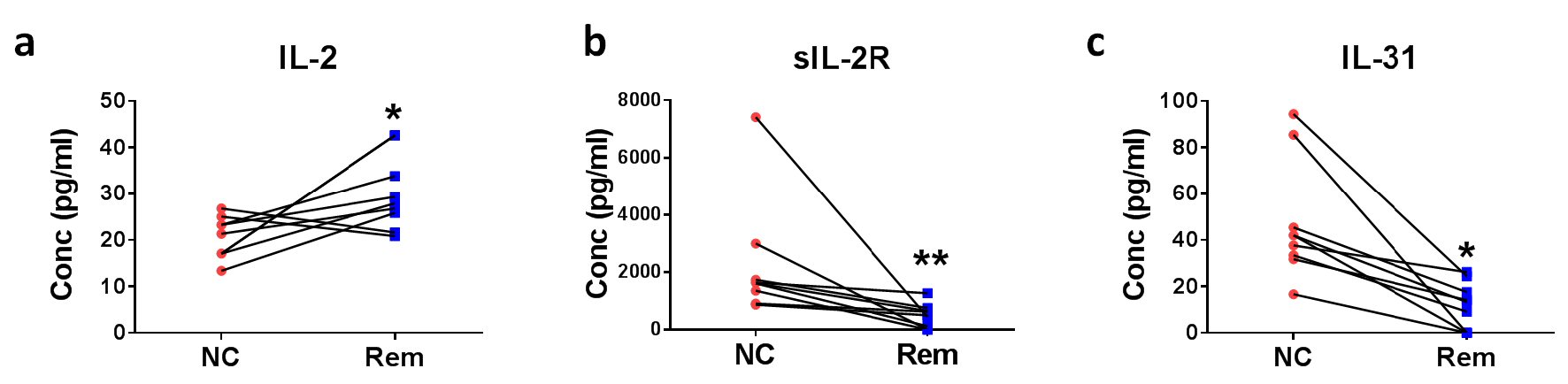 Click for large image | Figure 4. Soluble factors for the confirmation of remission status of DLBCL. Eight pairs of DLBCL patient plasmas before treatment (new cases (NC)) and after remission (Rem) were analyzed for (a) IL-2, (b) sIL-2R, and (c) IL-31 concentrations. *P < 0.05, **P < 0.01 (two-tailed, Wilcoxon matched-pairs signed rank test). |
| Discussion | ▴Top |
Raised plasma sCD30 level is an expected finding in cHL patients. CD30 is a surface marker on neoplastic HL that is expressed by the malignant Reed-Sternberg cells [12-14]. CD30 is currently utilized for immunohistochemical diagnosis of cHL to identify HRS cells. Serum sCD30 has also been found to be elevated in HL [12, 14] and has been associated with poor prognosis of HL patients [15, 16]. However, serum sCD30 is also elevated in many other inflammatory diseases (e.g. virus infections) and NHL (e.g. anaplastic large cell lymphoma (ALCL)) [17]. This widespread presence may explain why serum sCD30 is not being used for the diagnosis of cHL. Our study suggests that plasma sCD30 can differentiate cHL patients from DLBCL patients, the most common form of NHL. One limitation of our study however is the lack of data on the immunohistochemical CD30 status of the DLBCL cases included in the study. While CD30+ DLBCL is rare, it is plausible that plasma sCD30 might not have been able to differentiate these lymphomas from cHL.
CCL22 is a chemokine that interacts with CCR4, and attracts CCR4+ Th2 and Treg cells [18, 19]. In line with prior immunohistochemical studies which have shown that CCL22 levels are raised in cHL patients [18], CCL22 is also elevated in cHL patient plasma. Similar to sCD30, CCL22 is not specific to cHL. CCL22 has also been reported to be increased in other cancers such as nasal natural killer/T-cell lymphoma and breast cancer [20, 21]. Nevertheless, CCL22 has been shown to differentiate reliably between ALCL and cHL [22]. In addition, the difference in CCL22 levels between cHL and DLBCL patients is more prominent than the difference in sCD30 levels. Although CCL22 has been reported to be elevated in HTLV-1 carriers (median: 609 vs. 161 pg/mL, P < 0.0001) [23] and in EBV-caused infectious mononucleosis (mean: about 1,000 vs. 250 pg/mL) [24], the difference between cHL and healthy controls is more prominent (median: 619.6 vs. 67.0 pg/mL, P < 0.01). Therefore, while sCD30 is a good marker of lymphoma, given that sCD30 levels are elevated in 100% of the cHL patients and 90.9% of the DLBCL patients, CCL22 may be a better differentiating factor between cHL and DLBCL.
CXCL13 is a chemokine important in mediating the organisation of germinal centres in lymphoid tissue, and it is produced by CNS lymphoma cells [25] as well as by follicular lymphoma cells [26]. Due to the widespread presence of CXCL13 in other types of lymphomas, and the fact that the difference in CXCL13 levels between cHL patients and DLBCL patients is not pronounced, CXCL13 may not be a useful differentiating factor to definitely identify and diagnose cHL.
Our data show that plasma levels of sTNFR2 are lower in cHL patients in remission than in newly diagnosed cHL patients. In addition, when comparing plasma samples at time of initial diagnosis to those at remission of the same patients, levels of sTNFR2 were reduced too. This consistency shows that sTNFR2 may be of clinical use in verifying cHL remission.
TNFR2 is a membrane receptor which binds to TNF, a pro-inflammatory mediator [27]. It mediates a cell survival signal because in contrast to TNFR1, TNFR2 lacks a death domain [28]. A raised level of sTNFR2 is an adverse prognostic indicator for DLBCL, and predicts a poor response to therapy in HL and NHL [29]. However, so far no study proposed using sTNFR2 as a marker for remission of cHL. Plasma sTNFR2 could serve as a substitute for PET-CT to confirm remission which is expensive, radioactive, and requires specific infrastructure that may not be easily available at more remote healthcare centres. In contrast, measuring plasma cytokine levels is more convenient, more accessible and will likely reduce healthcare costs.
There are 19 more soluble factors that were significantly different between the cHL patient cohort at initial diagnosis and the cohort in remission. However, when comparing samples from the same patients at the time of diagnosis and during remission, levels of these soluble factors were not found to differ significantly, possibly due to small sample size (n = 8 pairs). APRIL and BAFF have been shown to enhance the survival as well as proliferation of HRS cells by delivering non-redundant signals via their receptors, TACI and BCMA [30]. Similarly, CD137 is expressed by HRS cells, and can dampen immune responses by trogocytosis [31, 32]. The plasma sIL-2R level during the disease phase is usually raised in CHL to mediate systemic immune suppression [33]. Furthermore, it has been proven in pediatric populations that sIL-2R decreases during therapy, and subsequently decreases further after the therapy is discontinued and patients are in remission [34].
Our results show that raised plasma IL-2 levels and decreased plasma levels of sIL-2R and IL-31 can potentially be used to confirm that DLBCL patients are in remission. There are limited data on the use of these cytokines in confirming remission of DLBCL patients. sIL-2R is known to be a poor prognostic factor for DLBCL patients as enhanced levels of sIL-2R are associated with lower rates of event-free survival and overall survival [35-37]. It will need to be shown in future studies whether there is a causal relationship between remission and the decreased sIL-2R concentration.
In summary, we propose to use the measurement of sCD30 and CCL22 in plasma as a minimally invasive alternative to the highly invasive biopsy method for diagnosis and screening of cHL. After cHL patients are treated, sTNFR2 may be used as a radiation-free alternative to PET-CT to evaluate patients’ response to the treatment, confirm the remission status of cHL, and potentially provide a novel way of follow-up for patients with cHL. These findings provide novel means for the clinical management of cHL, and could potentially lower the healthcare burden.
Conclusions
Measurement of soluble factors identified in this study may: 1) provide a minimally-invasive method to diagnose and differentiate HL and DLBCL, and 2) make it possible to monitor the remission status of these patients without the use of radiation-based imaging. Soluble CD30 and CCL22 could differentiate cHL patients from healthy controls and DLBCL patients. Soluble TNFR2 was reduced after cHL remission. IL-2 was elevated after DLBCL remission while sIL-2R and IL-31 were decreased.
Acknowledgments
We thank A. Mark Richards, Oi Wah Liew and Samantha Ling S.M. (Cardiovascular Research Institute, National University of Singapore, National University Health System) for assistance with the Bio-Plex® 200 System.
Financial Disclosure
This research was supported by a grant (OFIRG/0022/2016) from the National Medical Research Council, Singapore.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consents were obtained from the patients.
Author Contributions
The study is designed by HS. Acquisition of data by QZ and AG. Data analysis, statistics and interpretation of data by QZ. Drafting the article by QZ and HS, and revised critically for important intellectual content by AG, LX, MP. Final approval for submission by HS.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68(2):116-132.
doi pubmed - Kuppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol. 2005;37(3):511-517.
doi pubmed - Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221(3):248-263.
doi pubmed - Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol. 2019;184(1):45-59.
doi pubmed - Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(11):1574-1583.
doi pubmed - Renner C, Stenner F. Cancer Immunotherapy and the Immune Response in Hodgkin Lymphoma. Front Oncol. 2018;8:193.
doi pubmed - Pianko MJ, Liu Y, Bagchi S, Lesokhin AM. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig. 2017;4:32.
doi pubmed - Wahl RL, Kaminski MS, Ethier SP, Hutchins GD. The potential of 2-deoxy-2[18F]fluoro-D-glucose (FDG) for the detection of tumor involvement in lymph nodes. J Nucl Med. 1990;31(11):1831-1835.
- Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132(1):118-124.
- Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60(6):393-408.
doi pubmed - Gause A, Pohl C, Tschiersch A, Da Costa L, Jung W, Diehl V, Hasenclever D, et al. Clinical significance of soluble CD30 antigen in the sera of patients with untreated Hodgkin's disease. Blood. 1991;77(9):1983-1988.
- Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73(7):1349-1360.
doi - Vinante F, Morosato L, Siviero F, Nadali G, Rigo A, Veneri D, de Sabata D, et al. Soluble forms of p55-IL-2R alpha, CD8, and CD30 molecules as markers of lymphoid cell activation in infectious mononucleosis. Haematologica. 1994;79(5):413-419.
- Nadali G, Tavecchia L, Zanolin E, Bonfante V, Viviani S, Camerini E, Musto P, et al. Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin's disease at high risk of unfavorable outcome. Blood. 1998;91(8):3011-3016.
- Visco C, Nadali G, Vassilakopoulos TP, Bonfante V, Viviani S, Gianni AM, Federico M, et al. Very high levels of soluble CD30 recognize the patients with classical Hodgkin's lymphoma retaining a very poor prognosis. Eur J Haematol. 2006;77(5):387-394.
doi pubmed - Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10(6):457-470.
doi pubmed - Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, Jarrett RF, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140(5):527-536.
doi pubmed - Takegawa S, Jin Z, Nakayama T, Oyama T, Hieshima K, Nagakubo D, Shirakawa AK, et al. Expression of CCL17 and CCL22 by latent membrane protein 1-positive tumor cells in age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorder. Cancer Sci. 2008;99(2):296-302.
doi pubmed - Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, Durand I, et al. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71(19):6143-6152.
doi pubmed - Kumai T, Nagato T, Kobayashi H, Komabayashi Y, Ueda S, Kishibe K, Ohkuri T, et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. 2015;64(6):697-705.
doi pubmed - Doring C, Hansmann ML, Agostinelli C, Piccaluga PP, Facchetti F, Pileri S, Kuppers R, et al. A novel immunohistochemical classifier to distinguish Hodgkin lymphoma from ALK anaplastic large cell lymphoma. Mod Pathol. 2014;27(10):1345-1354.
doi pubmed - Toulza F, Nosaka K, Tanaka Y, Schioppa T, Balkwill F, Taylor GP, Bangham CR. Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3+ regulatory T cells. J Immunol. 2010;185(1):183-189.
doi pubmed - Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, Yoshie O. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus. J Virol. 2004;78(4):1665-1674.
doi pubmed - Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk HD, Cakiroglu H, Widmann T, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15(19):5968-5973.
doi pubmed - Husson H, Freedman AS, Cardoso AA, Schultze J, Munoz O, Strola G, Kutok J, et al. CXCL13 (BCA-1) is produced by follicular lymphoma cells: role in the accumulation of malignant B cells. Br J Haematol. 2002;119(2):492-495.
doi pubmed - Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416(6878):345-347.
doi pubmed - Vanamee ES, Faustman DL. TNFR2: A novel target for cancer immunotherapy. Trends Mol Med. 2017;23(11):1037-1046.
doi pubmed - Heemann C, Kreuz M, Stoller I, Schoof N, von Bonin F, Ziepert M, Loffler M, et al. Circulating levels of TNF receptor II are prognostic for patients with peripheral T-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18(13):3637-3647.
doi pubmed - Chiu A, Xu W, He B, Dillon SR, Gross JA, Sievers E, Qiao X, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109(2):729-739.
doi pubmed - Shao Z, Harfuddin Z, Pang WL, Nickles E, Koh LK, Schwarz H. Trogocytic CD137 transfer causes an internalization of CD137 ligand on murine APCs leading to reduced T cell costimulation. J Leukoc Biol. 2015;97(5):909-919.
doi pubmed - Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE, Moh MC, et al. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res. 2013;73(2):652-661.
doi pubmed - Chiu J, Ernst DM, Keating A. Acquired natural killer cell dysfunction in the tumor microenvironment of classic hodgkin lymphoma. Front Immunol. 2018;9:267.
doi pubmed - Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008;13(1):1-26.
doi pubmed - Yoshida N, Oda M, Kuroda Y, Katayama Y, Okikawa Y, Masunari T, Fujiwara M, et al. Clinical significance of sIL-2R levels in B-cell lymphomas. PLoS One. 2013;8(11):e78730.
doi pubmed - Hashimoto Y, Yokohama A, Saitoh A, Nakahashi H, Toyama K, Mitsui T, Koiso H, et al. Prognostic importance of the soluble form of IL-2 receptoralpha (sIL-2Ralpha) and its relationship with surface expression of IL-2Ralpha (CD25) of lymphoma cells in diffuse large B-cell lymphoma treated with CHOP-like regimen with or without rituximab: a retrospective analysis of 338 cases. J Clin Exp Hematop. 2013;53(3):197-205.
doi pubmed - Ennishi D, Yokoyama M, Terui Y, Asai H, Sakajiri S, Mishima Y, Takahashi S, et al. Soluble interleukin-2 receptor retains prognostic value in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP (RCHOP) therapy. Ann Oncol. 2009;20(3):526-533.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


