| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 2, June 2019, pages 71-78
Bone Marrow Findings of Immune-Mediated Pure Red Cell Aplasia Following Anti-Programmed Cell Death Receptor-1 Therapy: A Report of Two Cases and Review of Literature
Le Le Ayea, c, James B. Harrisb, Imran Siddiqia, Ashley Hagiyaa
aDepartment of Hematopathology, University of Southern California, Keck School of Medicine, Los Angeles, CA, USA
bDepartment of Pathology, Good Samaritan Hospital, Los Angeles, CA, USA
cCorresponding Author: Le Le Aye, Department of Hematopathology, University of Southern California, Keck School of Medicine, 1200 N. State Street, Room GNH 3840, Los Angeles, CA 90033, USA
Manuscript submitted May 3, 2019, accepted June 6, 2019
Short title: Immune-Mediated PCRA After Anti-PD-1
doi: https://doi.org/10.14740/jh507
| Abstract | ▴Top |
Immune checkpoint inhibitors have recently emerged as important and effective advanced cancer treatment options. Programmed cell death receptor-1 (PD-1) antagonists such as pembrolizumab and nivolumab have been approved by the US Food and Drug Administration for treatment of many advanced cancers. As anti-PD-1 checkpoint inhibitor use has been increasing, previously unreported rare side effects emerge. These checkpoint inhibitors upregulate humoral and cellular immune responses to tumor antigens. Consequently, they can be associated with immune-related adverse events including hematological-related reactions such as autoimmune hemolytic anemia, immune thrombocytopenia, neutropenia and pancytopenia. However, pure red cell aplasia (PRCA) induced by anti-PD-1 checkpoint inhibitors is rarely reported in the literature. We herein report cases of two patients who developed PRCA during treatment with anti-PD-1 checkpoint inhibitors. In both cases, a peripheral blood smear examination demonstrated reticulocytopenia. Bone marrow biopsies revealed severe erythroid hypoplasia with maturation arrest at the proerythroblast stage, relative granulocytic hyperplasia and lymphocytosis. Flow cytometry and immunohistochemistry revealed that the lymphocytes were predominantly CD8+ T cells. T lymphocytosis, especially in one of the two patients, mimicked a T-cell lymphoproliferative disorder; lack of clonality indicated a reactive process. Our findings, in addition to data presented in the literature, suggest that T cells play a critical role in the pathogenesis of immune-related PRCA. PRCA is an under-recognized immune-mediated adverse event that does not manifest during the clinical trial phase. It is a potentially life-threatening complication, which should be considered in the differential diagnosis of anemia in patients treated with anti-PD-1 checkpoint inhibitors.
Keywords: Pembrolizumab; Nivolumab; Immune checkpoint inhibitors; Immune-related adverse event; Pure red cell aplasia
| Introduction | ▴Top |
In recent years, manipulation of immune checkpoints has emerged as an important and effective cancer treatment strategy. Immune checkpoint inhibitors target cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1) and programmed cell death ligand-1 (PDL-1). The most widely studied and recognized immune checkpoint inhibitors are ipilimumab, pembrolizumab, nivolumab, atezolizumab, avelumab and durvalumab.
PD-1 is a negative costimulatory regulator that is expressed extensively in activated T lymphocytes and plays an important role in immunosurveillance mechanisms [1, 2]. Binding of PD-1 to its ligands PDL-1 and PDL-2 inhibits the cytotoxic T-cell response [3-5]. Anti-PD-1 checkpoint inhibitors block the interaction between the PD-1 on T cells and PDL-1 on tumor cells, restoring a functional cytotoxic tumor-specific T-cell response and leading to tumor cell death [6].
Anti-PD-1 checkpoint inhibitors are US Food and Drug Administration (FDA) approved for the treatment of many advanced cancers, namely melanoma, non-small cell lung cancer, urothelial cancer, renal cell carcinoma, head and neck cancer, classical Hodgkin lymphoma, gastric cancer, hepatocellular carcinoma and Merkel cell carcinoma [7]. In recent years, pembrolizumab and nivolumab have been approved by the FDA for the treatment of unresectable or metastatic, microsatellite instability-high or mismatch repair-deficient solid tumors such as metastatic colorectal cancer [8, 9]. As such, an increasing number of patients are exposed to anti-PD-1 drugs.
Common side effects include fatigue, pruritus, diarrhea, pyrexia, cough, dyspnea, musculoskeletal pain, constipation and nausea. Immune-mediated adverse events such as pneumonitis, colitis, hepatitis and thyroid disorder have been reported [10, 11]. With the broad use of anti-PD-1 drugs in clinical practice, rarer side effects are emerging. To date, hematological immune-related adverse events have been occasionally described, including immune thrombocytopenia [12], neutropenia [13], autoimmune hemolytic anemia [14, 15] and pancytopenia [16]. Pure red cell aplasia (PRCA), specifically, was not reported during clinical trials and has been rarely reported in the literature [17-19]. We herein describe the bone marrow findings of immune-mediated PRCA in the setting of anti-PD-1 checkpoint inhibitors.
| Case Reports | ▴Top |
Case 1
A 58-year-old woman was diagnosed with poorly differentiated squamous cell carcinoma of the right base of the tongue with lymph node metastases. She underwent partial glossectomy and right modified neck dissection in 2016. She was treated with five cycles of adjuvant radiation as well as 5-fluorouracil and cisplatin chemotherapy with weekly cetuximab postoperatively. Five months after surgery, she was found to have osseous metastatic lesions in T12 and L3 vertebral bodies with extension into the left pedicles and surrounding soft tissue extension into the left paraspinal muscles. Therefore, she was treated with palliative radiation therapy. Despite the extensive treatment, she was later found to have metastatic lesions in the liver. She then received two doses of immunotherapy with pembrolizumab.
Her initial hemoglobin level prior to treatment with pembrolizumab was 9.8 g/dL. Three weeks after receiving one dose of pembrolizumab, her hemoglobin dropped to 7.8 g/dL. She was subsequently transfused with one unit of packed red blood cells and received a second dose of pembrolizumab on the same day. She was noted to have isolated anemia with a hemoglobin of 6.7 g/dL three weeks after receiving the second dose. There was no known source of bleeding despite extensive medical workup. Direct antiglobulin test was negative, and total bilirubin was not elevated (1.1 mg/dL) with a direct bilirubin of 0.2 mg/dL. The differential diagnoses at that time included acquired drug-related PRCA, B19 parvovirus-associated aplastic crisis, a marrow infiltrative process such as squamous cell carcinoma metastasis to the bone marrow, or a lymphoproliferative disorder. To further evaluate the cause of anemia, she underwent a bone marrow biopsy and aspiration with a review of her peripheral blood smears. The bone marrow findings are consistent with those of PRCA, likely related to immunotherapy. Following the bone marrow biopsy, the patient was noted to have declining performance status and became more anemic. She was transferred to hospice care.
Case 2
The patient was a 74-year-old man with a history of liposarcoma of the leg who was treated with a 6-week course of radiation in April 2002. In March 2015, he noticed a growth over his left thigh; biopsy revealed moderately differentiated angiosarcoma. The patient underwent neoadjuvant chemotherapy with weekly Taxol, followed by local excision. In August 2017, he developed metastatic lesions in the liver; a biopsy confirmed angiosarcoma. Hence, he was diagnosed with stage IV angiosarcoma. He was initially treated with 13 weekly doses of Taxol and showed an excellent clinical and radiological response. Due to severe neuropathy, the treatment was switched to nivolumab on February 26, 2018. His baseline hemoglobin level prior to nivolumab was 12.7 g/dL. A computed tomography (CT) scan performed 1 month after receiving nivolumab showed improvement in all lesions, except for possible recurrence in the left lobe of the liver. Six months after therapy with nivolumab, the patient rapidly became anemic, with his hemoglobin dropping several grams every week to 6 - 7 g/dL. Despite receiving intravenous iron and Procrit, he continued to become severely anemic. A bone marrow biopsy was performed on September 12, 2018, for further evaluation. The patient was still on nivolumab at the time of the bone marrow biopsy. The bone marrow findings were consistent with those of PRCA, likely related to immunotherapy. Nivolumab therapy was discontinued and the patient was treated with a short course of glucocorticoids, which seemed to stabilize his hemoglobin level at 8 - 9 g/dL. His latest available hemoglobin level on January 18, 2019 was 7.7 g/dL after a packed red blood cell transfusion.
Pathological aspects of the reported cases
Complete blood count with differential and bone marrow aspirate differential counts are listed in Table 1. In both cases, peripheral blood smear examination showed marked normochromic normocytic anemia with marked reticulocytopenia, consistent with red cell aplasia. Granulocytic dysplasia, atypical lymphocytes, or blasts were not identified.
 Click to view | Table 1. Peripheral Blood and Bone Marrow Aspirate Differential Counts of Two Patients |
Significant findings from bone marrow examination were severe erythroid hypoplasia, relative granulocytic hyperplasia and lymphocytosis. Erythroblasts showed maturation arrest at the proerythroblast stage; polychromatic erythroblasts and orthochromatic erythroblasts were absent (Figs. 1a and 2a). Granulocytic maturation proceeded in an orderly fashion. There was no evidence of dysplasia. The myeloid/erythroid ratio had markedly increased (> 10:1). Megakaryocytes were morphologically unremarkable and adequate in number. The iron stain showed adequate iron stores; incorporation was present.
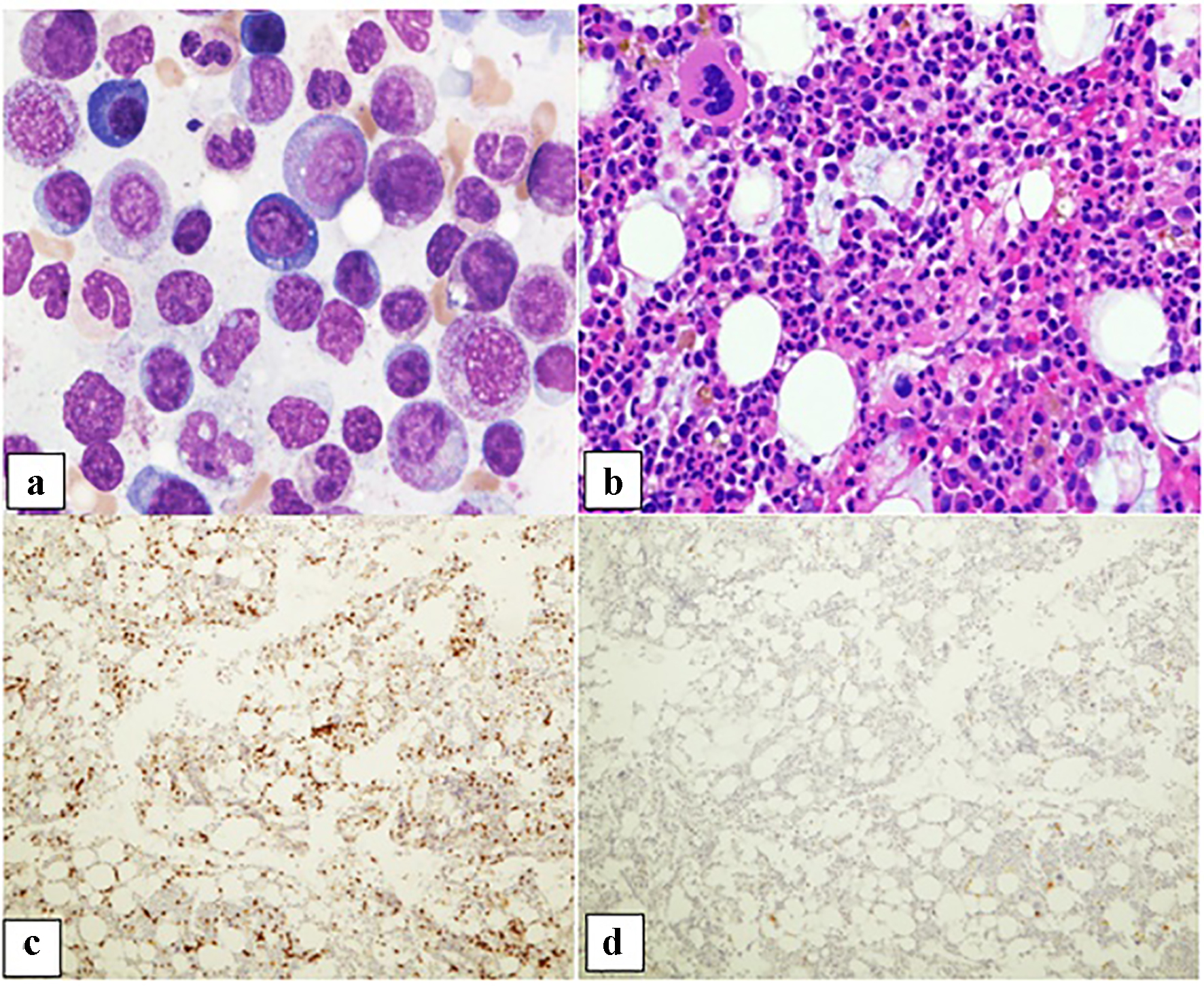 Click for large image | Figure 1. Patient 1. (a) Bone marrow aspirate smear demonstrating predominantly granulocytic precursors with rare pro-erythroblasts (× 50 oil objective). (b) Core biopsy showing a normocellular bone marrow with erythroid hypoplasia, granulocytic hyperplasia, and megakaryocytes with a normal appearance (× 20 objective). (c) CD3 immunohistochemical stain depicting increased scattered and occasional clusters of small lymphocytes (× 10 objective). (d) CD20 immunohistochemical stain with rare scattered small B lymphocytes (× 10 objective). |
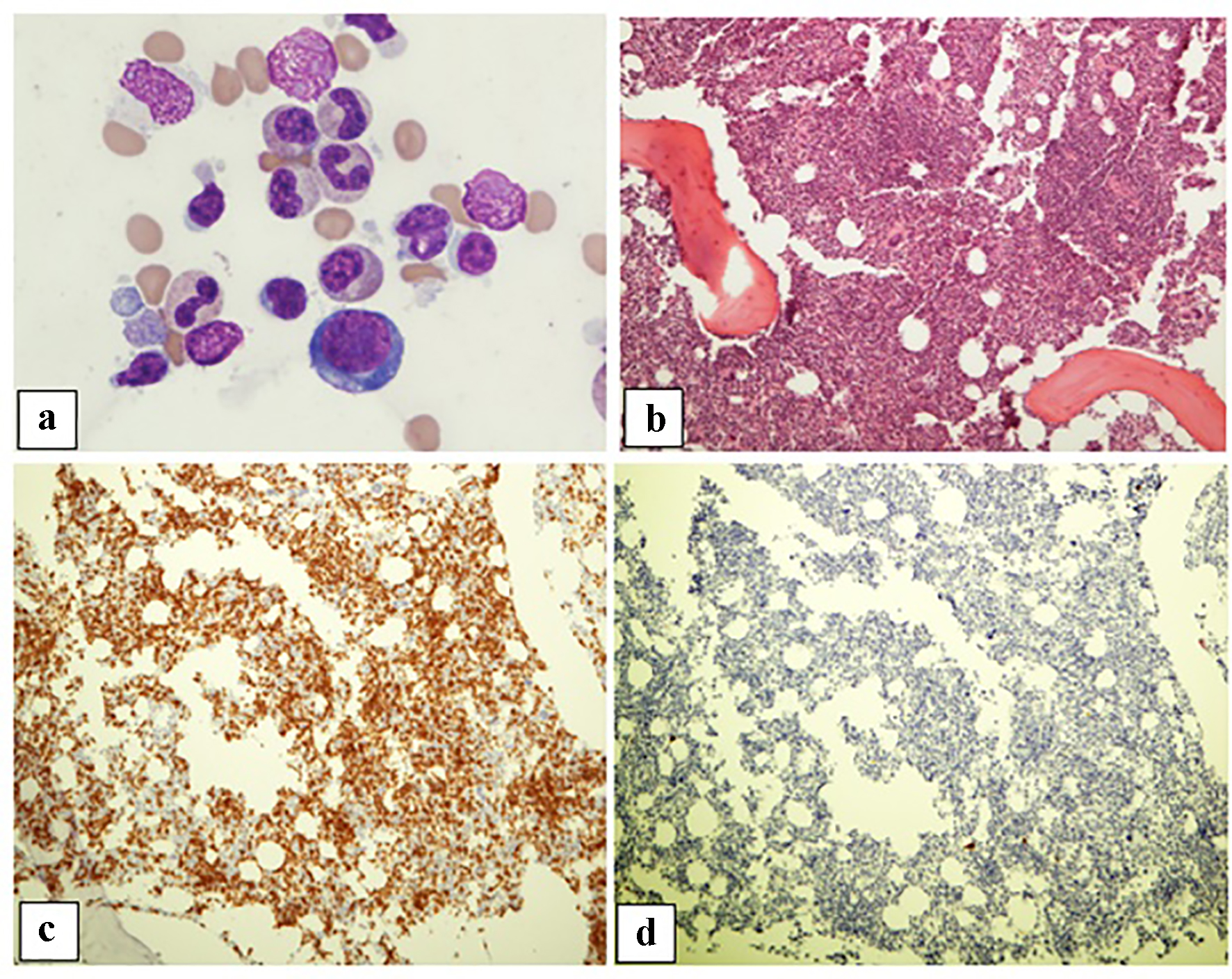 Click for large image | Figure 2. Patient 2. (a) Bone marrow aspirate showing rare pro-erythroblasts (× 50 oil objective). (b) Trephine biopsy showing a mildly hypercellular bone marrow with erythroid hypoplasia, granulocytic hyperplasia, megakaryocytes with a normal appearance, and increased lymphocytes without atypia. (c) CD3 immunohistochemical stains demonstrating increased T cells (× 10 objective). (d) CD20 immunohistochemical stain showing rare scattered small B cells (× 10 objective). |
The trephine biopsy sections showed normocellular bone marrow in case 1 (60% cellularity) and hypercellular bone marrow in case 2 (80% cellularity). Severe erythroid hypoplasia was confirmed (Figs. 1b and 2b). Notably, CD3 and CD20 immunohistochemical stains in both cases demonstrated numerous and occasional clusters of increased CD3+ T cells without cytologic atypia, far outnumbering CD20+ small B cells (Figs. 1c, 1d, 2c, 2d). Large intranuclear inclusions were not identified, and an immunohistochemical stain for parvovirus B19 was negative. There was no significant reticulin fibrosis (MF grade 0, scale 0-3 by European Consensus Classification) [20]. Immunohistochemical stain for the T-cell receptor bF1 antibody (TCR-bF1) performed in case 2 revealed increased alpha-beta T-cells, and TIA-1, indicating increased cytotoxic granules (Fig. 3c and d). Flow cytometry analysis performed on the marrow aspirate cells demonstrated reversed CD4/CD8 ratio (0.62 and 0.54 for case 1 and 2, respectively) (Fig. 4a and b). Case 1 showed increased gamma-delta T cells, which account for 20% of CD3+ lymphocytes (Fig. 4a).
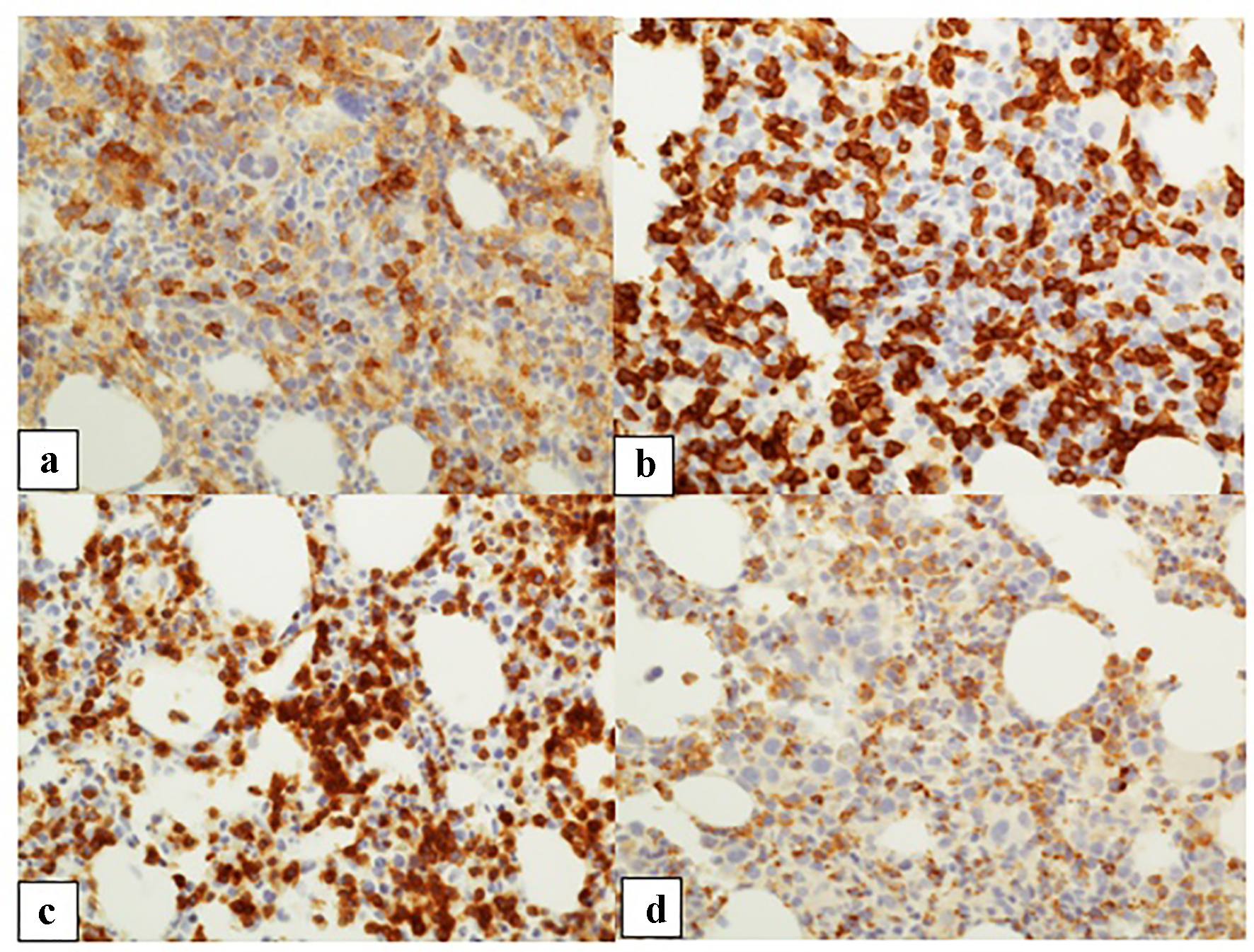 Click for large image | Figure 3. Patient 2. (a, b) CD4 and CD8 immunohistochemical stains, respectively (CD8 > CD4) (× 10 objective). (c) TCR bF1 staining highlighting a majority of T cells (× 10 objective). (d) T-cell intracytoplasmic antigen immunohistochemical stain highlighting many T cells (× 10 objective). |
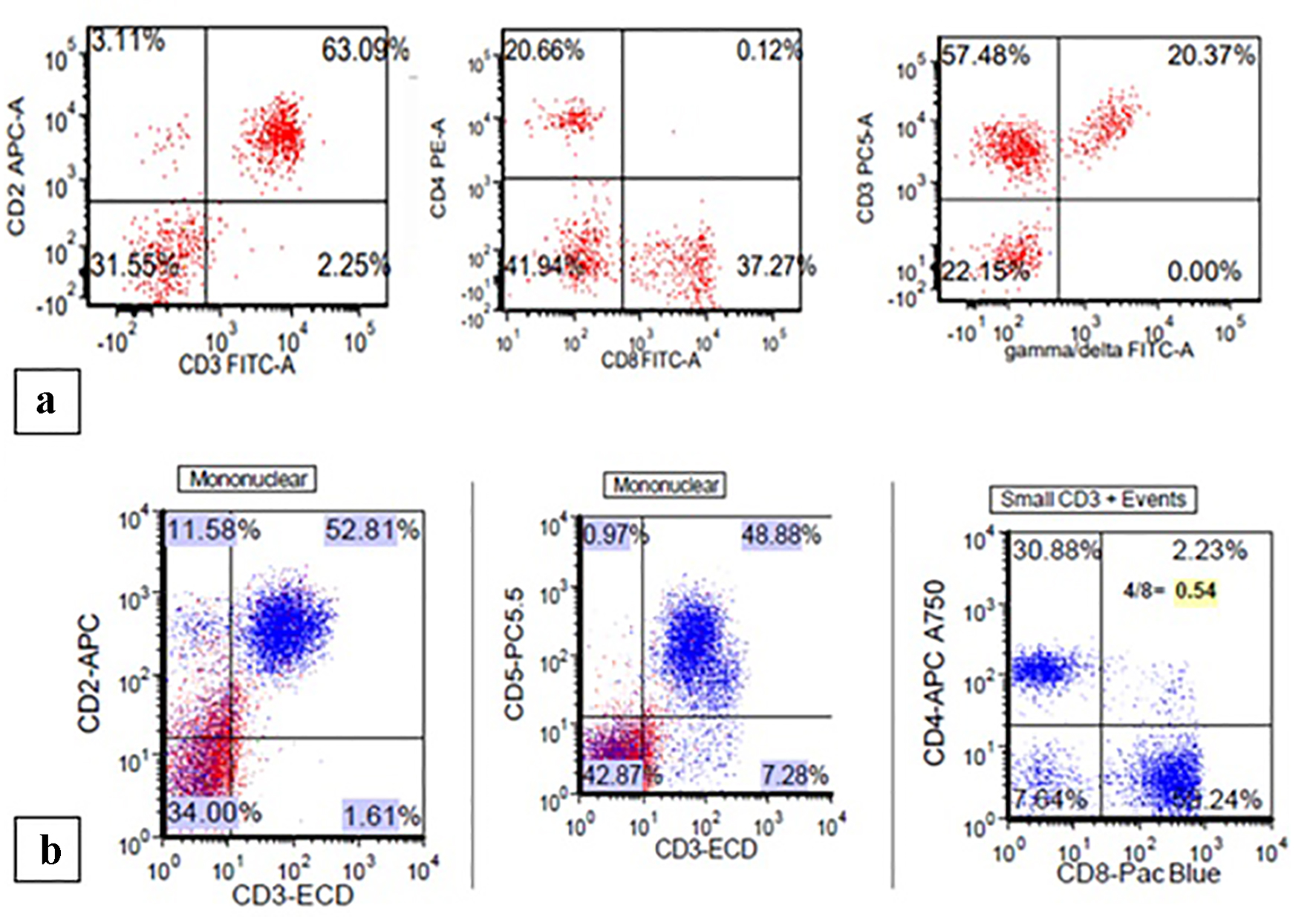 Click for large image | Figure 4. (a) Patient 1: flow cytometry demonstrating increased CD3+/CD8+ T cells with a CD4/CD8 ratio of 0.62 and increased gamma-delta T cells. (b) Patient 2: flow cytometry showing increased CD3+/CD8+ T cells with a CD4/CD8 ratio of 0.54. |
The moderate T lymphocytosis noted in case 2 mimicked a T-cell lymphoproliferative disorder; however, karyotyping revealed a normal male karyotype (46,XY[20]), and a T-cell receptor beta and gamma gene rearrangement study was negative. The overall findings are suggestive of PRCA, likely related to anti-PD-1 checkpoint inhibitors.
| Discussion | ▴Top |
PRCA is a disorder characterized by isolated normocytic normochromic anemia, reticulocytopenia and rare or absent erythroblasts (< 5% of erythroblasts on bone marrow differential count) in otherwise normal bone marrow [21, 22]. It can be classified as congenital PRCA (e.g. Diamond-Blackfan anemia) or acquired PRCA. Acquired PRCA may be associated with autoimmune/collagen vascular disorders; lymphoproliferative disorders such as chronic lymphocytic leukemia and large granular lymphocytic leukemia; solid tumors such as thymoma, gastric cancer, and renal cell carcinoma; infectious agents such as parvovirus; or drugs or chemicals-related reactions [22].
Immune cytopenia is considered a rare adverse event; it generally occurs within the first few months of starting immunotherapy, with an average onset time of 6 weeks [23]. PRCA associated with anti-PD-1 checkpoint inhibitors is extremely rare with very few cases reported in English literature [17-19]. Table 2 [17, 18, 24] shows the characteristics of patients with acquired PRCA in the setting of checkpoint inhibitor therapy, their bone marrow findings and treatment response. The time from the initial treatment with checkpoint inhibitors to the onset of anemia/PRCA can vary from 3 weeks to 21 months (Table 2). As such, the onset of anemia/PRCA can vary among patients, and a close clinical follow-up during treatment may be necessary.
 Click to view | Table 2. Characteristics and Bone Marrow Findings of Patients With Immune-Related Pure Red Cell Aplasia Following Checkpoint Inhibitor Therapy |
An increase in CD3+/CD8+ T cells noted in our two cases is believed to be a consequence of anti-PD-1 inhibitor use. This finding is in accord with those in reported cases of immunotherapy-related PRCA in literature [17, 24]. Studies have shown that patients with chronic viral infections and/or tumors have high PD-1 expression and blocking of the PD-1 pathway enhanced tumor-specific CD8+ T-cell response in vivo, resulting in increased T-cell proliferation, cytokine production, cytolytic activity and a reduction in viral load or tumor size [2]. In addition, T lymphocytosis, especially in patient 2, mimicked a T-cell lymphoproliferative disorder; a lack of T-cell clonality indicated an immune/inflammatory process.
Although the specific mechanisms of immune-related PRCA are not known, an increase in activated T cells is believed to play a critical role in pathogenesis. Erythropoiesis is the process by which hematopoietic stem cells proliferate and differentiate to produce mature red blood cells. It can be divided into two stages, early and late. During the early stage, hematopoietic stem cells give rise to burst-forming unit-erythroid and colony-forming unit-erythroid cells. Colony-forming unit-erythroid cells can be morphologically recognized as proerythroblasts. During the late stage, proerythroblasts undergo mitosis to produce basophilic, polychromatic and orthochromatic erythroblasts. Studies have shown that patients with acquired PRCA have increased gamma-delta T cells; recovery of PRCA is associated with a decrease in gamma-delta T cells [25, 26]. Experimental data have proven that gamma-delta T cells inhibit burst-forming and colony-forming unit-erythroid but not common myeloid progenitors [2]. Therefore, it seems reasonable to hypothesize that our bone marrow finding of marked erythroid hypoplasia with maturation arrest at the proerythroblast stage might be the result of the inhibitory effect by increased activated T cells. Further detailed mechanistic studies are needed to explore the triggers for a broader understanding of this rare immune-mediated phenomenon.
PRCA treatments including corticosteroids, cyclosporin A and intravenous immunoglobulin (IVIG) have shown variable responses [17-19, 27, 28]. Alemtuzumab, an anti-CD52 monoclonal antibody, is an alternative treatment option [29]. Although the 10-year survival rate is 80% or more with acquired PRCA, several deaths have been reported [28]. Therefore, PRCA is a potentially life-threatening complication and it is important to be aware of it. Patient 2 was noted to be anemic after discontinuation of the anti-PD-1 therapy, suggesting that the drug can have a long-lasting effect on patients.
Conclusion
In conclusion, we report the bone marrow findings of immune-mediated PRCA in the setting of anti-PD-1 checkpoint inhibitor use. PRCA is a rare immune-mediated adverse event that has not been reported during the clinical trial phase. Since there is no specific clinical presentation of PRCA besides signs and symptoms of anemia, the diagnosis requires a systematic approach including bone marrow examination, especially in patients with peripheral blood reticulocytopenia. PRCA is a potentially life-threatening complication and should be considered in the differential diagnosis of anemia in patients being treated with such agents.
Acknowledgments
Not applicable.
Financial Disclosure
Not applicable.
Conflict of Interest
Not applicable.
Informed Consent
Not applicable.
Author Contributions
LA and AH reviewed patient’s chart, performed research and wrote the manuscript. AH and IS diagnosed the patient 1 bone marrow and JH diagnosed the patient 2 bone marrow. All authors read and approved the final manuscript.
Abbreviations
PRCA: pure red cell aplasia; PD-1: programmed cell death receptor-1; CTLA-4: cytotoxic T lymphocyte-associated molecule-4; PDL-1: programmed cell death ligand-1; WBC: white blood cell; Hb: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; PLT: platelets
| References | ▴Top |
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116-126.
doi pubmed - Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
doi pubmed - Freeman GJ. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A. 2008;105(30):10275-10276.
doi pubmed - Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-1034.
doi pubmed - Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793-800.
doi pubmed - Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207-212.
doi pubmed - Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8.
doi pubmed - Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019.
doi - Mehrvarz Sarshekeh A, Overman MJ, Kopetz S. Nivolumab in the treatment of microsatellite instability high metastatic colorectal cancer. Future Oncol. 2018;14(18):1869-1874.
doi pubmed - Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-485.
doi pubmed - Hsu JC, Lin JY, Hsu MY, Lin PC. Effectiveness and safety of immune checkpoint inhibitors: A retrospective study in Taiwan. PLoS One. 2018;13(8):e0202725.
doi pubmed - Le Roy A, Kempf E, Ackermann F, Routier E, Robert C, Turpin A, Marabelle A, et al. Two cases of immune thrombocytopenia associated with pembrolizumab. Eur J Cancer. 2016;54:172-174.
doi pubmed - Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508.
doi - Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MS. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res. 2016;26(2):202-204.
doi pubmed - Inadomi K, Kumagai H, Arita S, Tsuruta N, Takayoshi K, Mishima K, Ota S, et al. Bi-cytopenia possibly induced by anti-PD-1 antibody for primary malignant melanoma of the esophagus: A case report. Medicine (Baltimore). 2016;95(29):e4283.
doi pubmed - Atwal D, Joshi KP, Ravilla R, Mahmoud F. Pembrolizumab-induced pancytopenia: a case report. Perm J. 2017;21:17-004.
doi pubmed - Nair R, Gheith S, Nair SG. Immunotherapy-Associated Hemolytic Anemia with Pure Red-Cell Aplasia. N Engl J Med. 2016;374(11):1096-1097.
doi pubmed - Yuki A, Takenouchi T, Takatsuka S, Ishiguro T. A case of pure red cell aplasia during nivolumab therapy for cardiac metastatic melanoma. Melanoma Res. 2017;27(6):635-637.
doi pubmed - Davis EJ, Salem JE, Young A, Green JR, Ferrell PB, Ancell KK, Lebrun-Vignes B, et al. Hematologic complications of immune checkpoint inhibitors. Oncologist. 2019;24(5):584-588.
doi pubmed - Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128-1132.
- Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142(4):505-514.
doi pubmed - Means RT, Jr. Pure red cell aplasia. Blood. 2016;128(21):2504-2509.
doi pubmed - Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo). 2013;2013:857519.
doi pubmed - Gordon IO, Wade T, Chin K, Dickstein J, Gajewski TF. Immune-mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol Immunother. 2009;58(8):1351-1353.
doi pubmed - Liu M, Liu T, Meng WT, Zhu HL, Cui X. [Role of gammadeltaT cells in pathogenesis of acquired pure red cell aplastic anemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15(1):142-146.
- Chinello M, Mauro M, Cantalupo G, Balter R, De Bortoli M, Vitale V, Zaccaron A, et al. Pure Red Cell Aplasia (PRCA) and Cerebellar Hypoplasia as Atypical Features of Polyglandular Autoimmune Syndrome Type I (APS-1): two sisters with the same AIRE mutation but different phenotypes. Front Pediatr. 2019;7:51.
doi pubmed - Adachi M, Yoshida K, Shiraishi Y, Chiba K, Miyano S, Ogawa S. [Successful treatment of pure red cell aplasia with cyclosporin in a patient with T-cell large granular lymphocytic leukemia harboring the STAT3 D661V mutation]. Rinsho Ketsueki. 2019;60(1):39-45.
- Ru X, Liebman HA. Successful treatment of refractory pure red cell aplasia associated with lymphoproliferative disorders with the anti-CD52 monoclonal antibody alemtuzumab (Campath-1H). Br J Haematol. 2003;123(2):278-281.
doi pubmed - Sawada K, Hirokawa M, Fujishima N, Teramura M, Bessho M, Dan K, Tsurumi H, et al. Long-term outcome of patients with acquired primary idiopathic pure red cell aplasia receiving cyclosporine A. A nationwide cohort study in Japan for the PRCA Collaborative Study Group. Haematologica. 2007;92(8):1021-1028.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


