| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 1, Number 4-5, October 2012, pages 95-99
Transfusion-Related Acute Lung Injury With Fatal Outcome
Trine Korsholma, b, Lone Winther Lietzena
aDepartment of Clinical Immunology, Aarhus University Hospital, Skejby, Denmark
bCorresponding author: Trine Korsholm, Department of Clinical Immunology, Aarhus University Hospital, Skejby, Brendstrupgaardsvej 100, 8200 Aarhus N, Denmark
Manuscript accepted for publication August 2, 2012
Short title: Transfusion-Related Acute Lung Injury
doi: https://doi.org/10.4021/jh38e
| Abstract | ▴Top |
Transfusion-related acute lung injury (TRALI) is a life-threatening complication of blood transfusion and has emerged as a leading cause of transfusion-associated morbidity and mortality. Prospective data of patients in the intensive care units have documented a significantly higher incidence of TRALI than previously reported. TRALI is likely to be under diagnosed due to a lack of awareness and understanding of this clinical entity, and because of the clinical presentation mirroring acute respiratory distress syndrome with its various potential aetiologies. We present a case of fatal TRALI in a 61-year-old man, who exhibited acute onset of respiratory distress postoperatively in association with transfusion of fresh frozen plasma (FFP) containing human leukocyte antigen (HLA) antibodies reactive with patient HLA. The patient had just been pneumonectomied. Risk factors of the blood component as well as patient specific risk factors determining the risk of TRALI are discussed. There is a need to increase the awareness and reporting of this diagnosis, in part to promptly institute the appropriate treatment and in part to identify and defer donors implicated in TRALI. Furthermore, awareness of this type of complication to transfusion of blood products could increase rational use of blood products, including minimising the use of FFP as volume therapy.
Keywords: TRALI; Transfusion; Transfusion complication
| Introduction | ▴Top |
Transfusion-related acute lung injury (TRALI) is a leading cause of transfusion-associated mortality. The link between symptoms of acute lung injury (ALI), transfusion and leukoagglutinins was first reported by Brittingham in 1957 [1], and in 1983 the term TRALI was introduced [2]. The April 2004 Canadian Consensus Conference proposed diagnostic criteria of TRALI, which are summarised in Figure 1 [3].These are based primarily on clinical and radiographic findings. Signs and symptoms include acute onset of respiratory distress, hypoxemia, frothy pulmonary secretions, hypotension (less commonly hypertension), fever, tachycardia and occasional cyanosis [1, 2]. Radiographic examination of the chest shows bilateral fluffy infiltrates or complete 'whiteout' of the entire lung fields [1, 4]. Unless there is an underlying cardiac condition or concomitant fluid overload, there is no evidence of circulatory overload with absence of jugular venous distension or a cardiac gallop, and central venous pressure as well as pulmonary capillary wedge pressure are normal [1]. All blood components have been implicated in TRALI; however, plasma-rich blood components are most commonly implicated with fresh frozen plasma (FFP) and apheresis platelets having caused the largest number of reported cases [5].
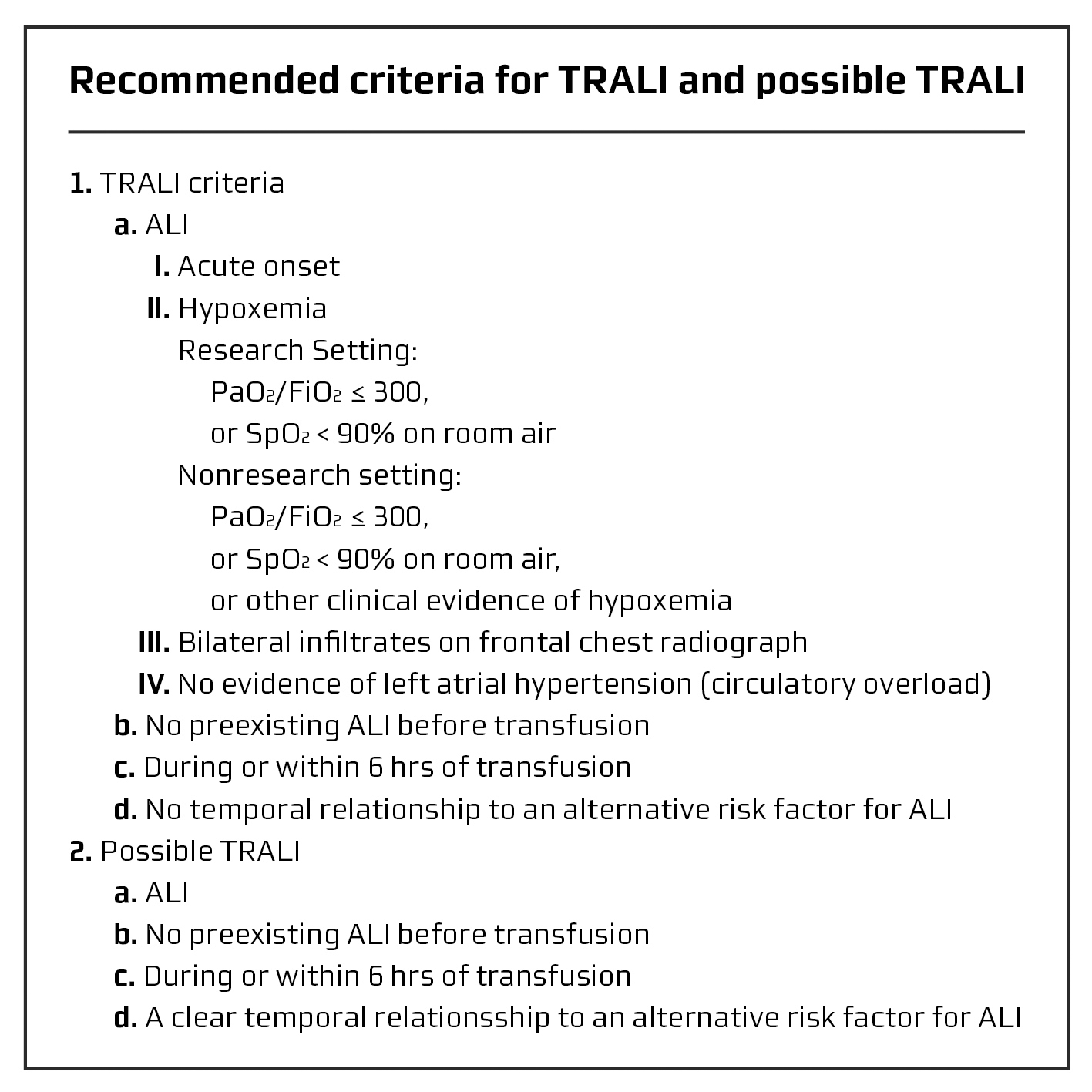 Click for large image | Figure 1. Proposed diagnostic criteria of TRALI. Adapted with permission from Kleinman et al [3] |
The exact pathophysiological mechanism(s) for TRALI are not yet fully understood, however, there is strong clinical and experimental evidence of an antibody-mediated mechanism in which passive transfer of white blood cell (WBC) antibodies with a transfusion of plasma-containing blood components results in binding to recipient neutrophils. Neutrophils are activated and sequestered in lung capillaries where complement activation and release of neutrophil bioactive products causes endothelial damage, capillary leak and ALI [1]. WBC antibodies are directed against human leukocyte antigens (HLA) or human neutrophil antigens (HNA). These alloantibodies arise in individuals lacking the antigens on their own cells, who are exposed to cells containing the antigen. The most frequent situation for this to occur is during pregnancy, where the mother is exposed to foetal cells expressing the alloantigen inherited from the father. Donor and patient HLA and/or HNA incompatibility is not part of the criteria (Fig. 1), but evidence of HLA/HNA incompatibility strongly supports the diagnosis. An alternative ‘two-event model’ has been postulated and well documented by Silliman et al [6]. The clinical condition of the patient, including inflammation, surgery and infection as the first ‘event’ cause priming of the neutrophils. The second ‘event’ is neutrophil activation caused by transfused antibodies or biologically active substances accumulated in the blood component such as lipids and CD40 ligand [6].
TRALI is a life-threatening complication of blood transfusion. It is likely to be under diagnosed due to a lack of awareness and understanding of this clinical entity, and because of the clinical presentation mirroring acute respiratory distress syndrome with its various potential aetiologies.
We present a case of clear-cut TRALI with a fatal outcome.
| Case Report | ▴Top |
A 61-year-old man previously healthy with newly discovered lung cancer, was admitted to thoracotomy for lobotomy or pneumonectomy. The right lung was removed by surgery. The perioperative course was uncomplicated and the patient was extubated in the operating room prior to admission to the intensive care unit (ICU). Postoperatively the patient presented oozing and was transfused with FFP. During transfusion of the second unit of FFP, the patient exhibited acute respiratory distress, and he became cold and sweating on extremities. Concurrently, the arterial oxygen saturation and blood pressure fell and heart rate increased (Fig. 2). Shortly before the onset of respiratory distress, a routine chest X-ray (X-ray # 1) (Fig. 3) was performed showing normal postoperative conditions for the remaining left lung. 25 minutes later (20 minutes after the onset of respiratory distress), a new chest X-ray (X-ray # 2) (Fig. 3) showed consolidation of the remaining left lung, described by the radiologist to be indicative of acute intra-alveolar pulmonary oedema. ECG displayed sinus tachycardia with no signs of myocardial ischemia. Echocardiography showed good biventricular function. The left ventricle was without dilation or regional dyskinesia and with normal flow towards left side heart valves. Right side cavities appeared normal and there were no pericardial exudates. Thus, there were no signs of cardiogenic shock. Bronchoscopy was performed acutely and showed no bronchial leakage. Bloody, yellowish, liquid discharge increased into the drainage tube indicating plasma effusion.
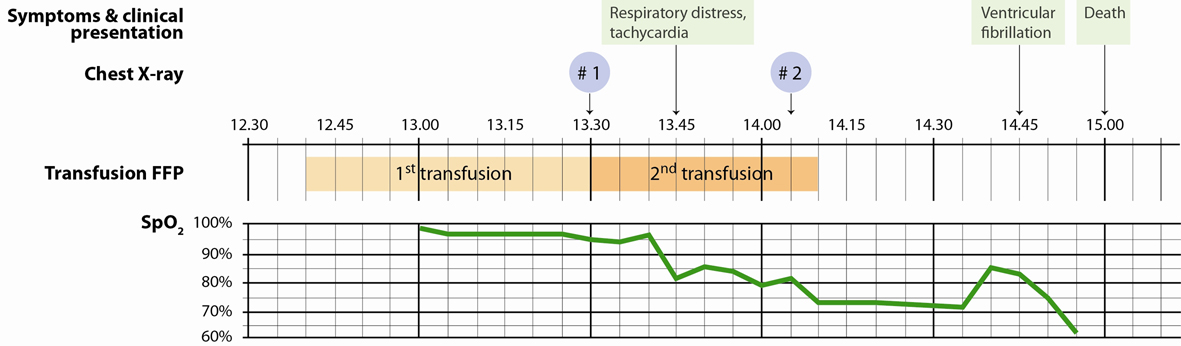 Click for large image | Figure 2. Time course of symptoms, clinical presentation including SpO2 and timing of chest X-rays in relation to transfusions of FFP. |
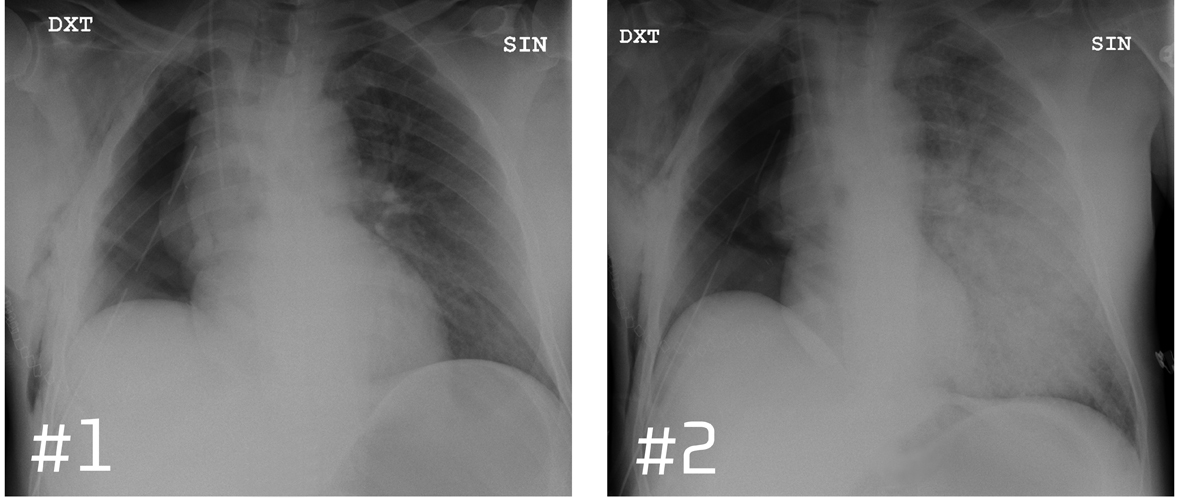 Click for large image | Figure 3. Chest X-ray before and after the onset of respiratory distress. |
Despite all efforts to stabilize the patient with inotropic support and assisted ventilation, the circulation collapsed and the patient had asystoli. In spite of attempts of resuscitation, he died 90 minutes after the start of the second FFP transfusion. Legal post mortem examination determined the cause of death to be acute lung failure. Surgical lines were found intact and judged to be perfect. Exudates were found in the bronchial airways in both chest cavities and thickening of the right ventricle was observed.
Methods
The HLA type of the patient, HLA antibody identification in donors and WBC cross match between the patient and each donor were performed with a standard complement dependent cytotoxicity technique.
HLA typing: Lymphocytes from the patient are tested against a panel of selected HLA antibodies with known specificity. Rabbit serum is added as a source of complement. If antibodies have bound to cognate antigens of the lymphocytes, complement will be activated and the cells killed. This is visualized by staining followed by flourescence microscopic evaluation.
HLA antibody identification: Serum from the patient and relevant donors are tested against a selected panel of lymphocytes from HLA-typed individuals.
Cross match: Donors sera were tested for reactivity against the patient's lymphocytes.
Results
In donor #1 no HLA antibodies were identified and cross match between serum from donor #1 and patient lymphocytes was negative. In donor #2 polyspecific HLA antibodies were identified. Cross match between serum from donor #2 and patient lymphocytes was strongly positive showing that the donor’s serum contained HLA antibodies directed at patient’s lymphocytes.
Investigation of HNA antibodies was not performed.
| Discussion | ▴Top |
Based on the symptoms of acute respiratory distress, the clinical findings of hypoxemia, non-cardiogenic pulmonary oedema accompanied by the sudden emergence of infiltrates in the remaining lung on chest X-ray and the clear temporal relationship to the infusion of FFP, TRALI is highly likely. The symptoms start abruptly 25 minutes after the initiation of transfusion with FFP from donor #2 and there is no other apparent cause. The findings of WBC antibodies in donor #2 recognizing cognate antigen(s) on patient lymphocytes in the WBC cross match further support the diagnosis of TRALI. Differential diagnosis of TRALI requires consideration of other patient-related causes of acute respiratory insufficiency, such as cardiac failure, circulatory overload, and in this particular case the possibility of an anastomotic leak with bleeding into the chest cavity as well as cardiac herniation. Echocardiography performed shortly after the debut of symptoms showed good biventricular function with no signs of left or right heart failure and no cardiac herniation. Bronchoscopy revealed no bronchial lesion or bleeding, but considerable amounts of yellow fluid indicating plasma leakage.
The rapid and severe course of TRALI in our patient could be due to the circumstance that the transfusion was given in a central venous catheter: consequently with almost no dilution of the plasma before recipient-specific antibodies met surgery-induced primed neutrophils and endothelium in the pulmonary capillary bed. There is also the possibility of additional recipient-directed alloantibodies in the culprit component of FFP. Alloantibodies directed against HNA-3a have been repeatedly reported to cause severe and fatal TRALI [7, 8]. Unfortunately, analysis of donor anti-HNA antibodies was not performed and we can only speculate on their existence in this case. The culprit component of FFP came from a multiparous woman. Among blood donors, HLA antibodies are found more frequently in multiparous women [9]. In 2003 the UK began a TRALI risk reduction effort converting to the use of predominantly male plasma, resulting in a significant risk reduction of highly likely/probable TRALI due to FFP [10]. In 2006 American Red Cross followed this trend and began to distribute plasma preferentially from male donors with a significantly decrease in TRALI cases [11]. The same preventive measure was taken in our transfusion service months prior to the incident, but to ensure supply continuity, FFP from female donors of type AB was not withdrawn from the freezers. Other preventive measures to reduce antibody-mediated TRALI include WBC antibody screening (HLA and HNA antibodies) of blood donors as suggested by The International Society of Blood Transfusion (ISBT) [12]. Two recent studies [7, 13] have by two different approaches uncovered the nature of the HNA-3a antigen, thereby providing the basis for high-throughput screening-assays using recombinantly expressed HNA-3a antigen to detect these potentially detrimental alloantibodies in blood donors. In addition to risk factors of the blood component, patient-specific risk factors determine the probability of TRALI. One could propose the pneumonectomy to be a risk factor for acute lung injury in our patient: As reviewed by Silliman [6] and Bux [14] surgery can lead to priming of neutrophils. Priming results in ‘stiffening’ of the cell and possibly mechanical retention in the pulmonary capillary bed, prolonging the close contact of the primed neutrophil with endothelial surface [14]. Primed and sequestered neutrophils can then be activated by exogenous activating factors such as WBC antibodies in donor blood to express full microbicidal activity leading to endothelial cell damage and pulmonary oedema.
TRALI is an under-diagnosed and under-reported clinical event in transfused patients. The reported incidence is highly variable owing to various study designs and characteristics of patient populations, and lack of diagnostic criteria before 2004. The true incidence of TRALI is unknown. Critically ill patient groups appear to have a higher risk of TRALI. Recent prospective data of patients in the ICU’s have documented a significantly higher incidence of TRALI (8%) than previously reported [15]. In critically ill patients the presentation of respiratory distress is common and has several possible underlying aetiologies, and therefore less likely to be recognised as TRALI in spite of a clear temporal relationship to transfusion. Epidemiological studies found transfusion to be the most common predisposing factor for acute respiratory distress syndrome [16, 17]. Several of these could likely be unrecognised cases of TRALI. Once suspected, TRALI case ascertainment is more obvious in ICU’s than other departments due to their ability to link several real-time databases that contain the actual timing of events such as transfusion and alteration in respiratory function [18]. Especially in the ICU’s, there is a need to increase the awareness and reporting of this diagnosis, in part to promptly institute the appropriate treatment and in part to identify and defer donors implicated in TRALI. Furthermore, awareness of this type of complication to transfusion of blood products could increase rational use of blood products, including minimising the use of FFP as volume therapy. Having said that, further research is needed to determine the optimal transfusion strategies in the various groups of critically ill patients.
The treatment of TRALI is supportive: primarily assisted ventilation with supplemental oxygen. Measures to minimize time on mechanical ventilation could improve outcome [19]. A potential effect of steroids have not been proven and diuretics may cause decreases in intravascular volume and are not indicated [1, 5]. Transfusions have been shown to worsen the outcome in patients with existing ALI [4], calling for a restrictive transfusion strategy in these patients. Prospective randomized controlled trials involving intensive care physicians and transfusion medicine experts would contribute valuable knowledge of the true incidence of TRALI, transfusion and patient-specific risk factors as well as assessment of the true effect of various preventive measures.
Acknowledgments
We are grateful to Niels Grunnet, MD, DMSc, for valuable intellectual contributions as well as general support.
Conflicts of Interest
None.
Research Support Funding
None.
| References | ▴Top |
- Triulzi DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108(3):770-776.
pubmed doi - Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983;128(1):185-189.
pubmed - Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44(12):1774-1789.
pubmed doi - Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004;126(1):249-258.
pubmed doi - Silliman CC, Fung YL, Ball JB, Khan SY. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Rev. 2009;23(6):245-255.
pubmed doi - Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124-131.
pubmed doi - Greinacher A, Wesche J, Hammer E, Furll B, Volker U, Reil A, Bux J. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16(1):45-48.
pubmed doi - Bux J. Human neutrophil alloantigens. Vox Sang. 2008;94(4):277-285.
pubmed doi - Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825-1835.
pubmed doi - Chapman CE, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452.
pubmed doi - Eder AF, Herron RM, Jr., Strupp A, Dy B, White J, Notari EP, Dodd RY, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006-2008). Transfusion. 2010;50(8):1732-1742.
pubmed doi - Bierling P, Bux J, Curtis B, Flesch B, Fung L, Lucas G, Macek M, et al. Recommendations of the ISBT Working Party on Granulocyte Immunobiology for leucocyte antibody screening in the investigation and prevention of antibody-mediated transfusion-related acute lung injury. Vox Sang. 2009;96(3):266-269.
pubmed doi - Curtis BR, Cox NJ, Sullivan MJ, Konkashbaev A, Bowens K, Hansen K, Aster RH. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood. 2010;115(10):2073-2076.
pubmed doi - Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol. 2007;136(6):788-799.
pubmed doi - Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886-891.
pubmed doi - Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59(3):717-723.
pubmed - Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, Petty TL, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593-597.
pubmed - Kleinman S. A perspective on transfusion-related acute lung injury two years after the Canadian Consensus Conference. Transfusion. 2006;46(9):1465-1468.
pubmed doi - Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126-134.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


