| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 2, June 2019, pages 79-82
Herpes Simplex Necrotic Lymphadenitis Masquerading as Richter’s Transformation in Treatment-Naive Patients With Chronic Lymphocytic Leukemia
Yan Amber Hodgsona, d, Stephen Gareth Jonesb, Helen Knighta, Vishakha Sovanic, Christopher Paul Foxa
aClinical Haematology, Nottingham University Hospitals NHS Trust (City Campus), Hucknall Rd, Nottingham, NG5 1PB, UK
bClinical Haematology, King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust, Mansfield Road, Sutton-in-Ashfield, NG17 4JL, UK
cHistopathology, Nottingham University Hospitals NHS Trust (City Campus), Hucknall Rd, Nottingham, NG5 1PB, UK
dCorresponding Author: Yan Amber Hodgson, Clinical Haematology, Nottingham University Hospitals NHS Trust (City Campus), Hucknall Rd, Nottingham, NG5 1PB, UK
Manuscript submitted May 12, 2019, accepted June 7, 2019
Short title: HSV Lymphadenitis Mimicking Richter’s Transformation
doi: https://doi.org/10.14740/jh517
| Abstract | ▴Top |
Richter’s transformation from chronic lymphocytic leukemia (CLL) to aggressive lymphoma is a relatively rare event with well-characterised clinical and radiological features, and can typically be distinguished from infectious complications of CLL. Opportunistic infections in CLL happen predominantly in the context of immunosuppressive therapy and/or relapsed/refractory disease. Herpes simplex viral (HSV) lymphadenitis is a rare phenomenon in treatment-naive CLL patients with only six cases reported in the English-language literature. Its diagnosis is challenging; its management and the outcome of the subsequent treatment for CLL are not well documented. We report three cases of rapidly progressive lymphadenopathy occurring in the context of previously untreated CLL, clinically and radiologically mimicking Richter’s transformation, but histologically confirmed as necrotic HSV lymphadenitis. We describe the presentation, diagnosis and management of all three cases, including for the first time the positron emission tomography-computed tomography (PET-CT) appearance of this condition, as well as how we later on delivered CLL-directed immunochemotherapy safely and successfully without recrudescence of HSV-related disease. Our cases underscore the importance of obtaining biopsy in all cases of rapidly progressive or disconcordant lymphadenopathy in CLL patients, or in those with highly 18FDG-avid adenopathy on PET-CT.
Keywords: Small lymphocytic leukemia; Chronic lymphocytic leukemia; Lymphadenopathy; Herpes simplex; Lymphadenitis; HSV; Lymphoma; Lymphoproliferative diseases
| Introduction | ▴Top |
Richter’s transformation is a relatively rare complication of chronic lymphocytic leukemia (CLL) and/or small lymphocytic lymphoma (SLL) characterised by rapid growth of a nodal or extra-nodal mass, with histological evidence of aggressive lymphoma (DLBCL). Clinical management of Richter’s transformation requires a high index of clinical suspicion, rapid diagnostic confirmation and urgent treatment with immunochemotherapy. Unfortunately, resistance to conventional treatment is common and a poor prognosis is anticipated for a majority of patients. Herpes simplex viral (HSV) lymphadenitis is an extremely rare condition that can occur as a localised infection or as part of systemic viral dissemination with less than 30 cases reported worldwide [1-3], the first of which was in France in 1991 [4]. A total of 15 cases were described in the English-language literature, of which six cases were treatment-naive patients. We report three cases of necrotising HSV lymphadenitis masquerading as Richter’s transformation in treatment-naive CLL patients.
| Case Reports | ▴Top |
Case 1
A 65-year-old lady with treatment-naive CLL, on a watch and wait policy for 12 years since diagnosis, presented with a short history of progressive, bulky cervical lymphadenopathy. There was no evidence of concurrent systemic infection. Clinical examination revealed a large firm mass in the right neck with a smaller fluctuant swelling on the left side of the neck with overlying erythema, and further submandibular adenopathy. Richter’s transformation to aggressive lymphoma was suspected clinically and she underwent whole body imaging with positron emission tomography-computed tomography (PET-CT) which demonstrated highly fludeoxyglucose (FDG)-avid extensive confluent bilateral lymphadenopathy in the neck together with a soft tissue mass in the right palatine tonsil (Fig. 1). The imaging findings supported the clinical suspicion of Richter’s transformation although the serum lactate dehydrogenase (LDH) was noted to be within the normal laboratory reference range.
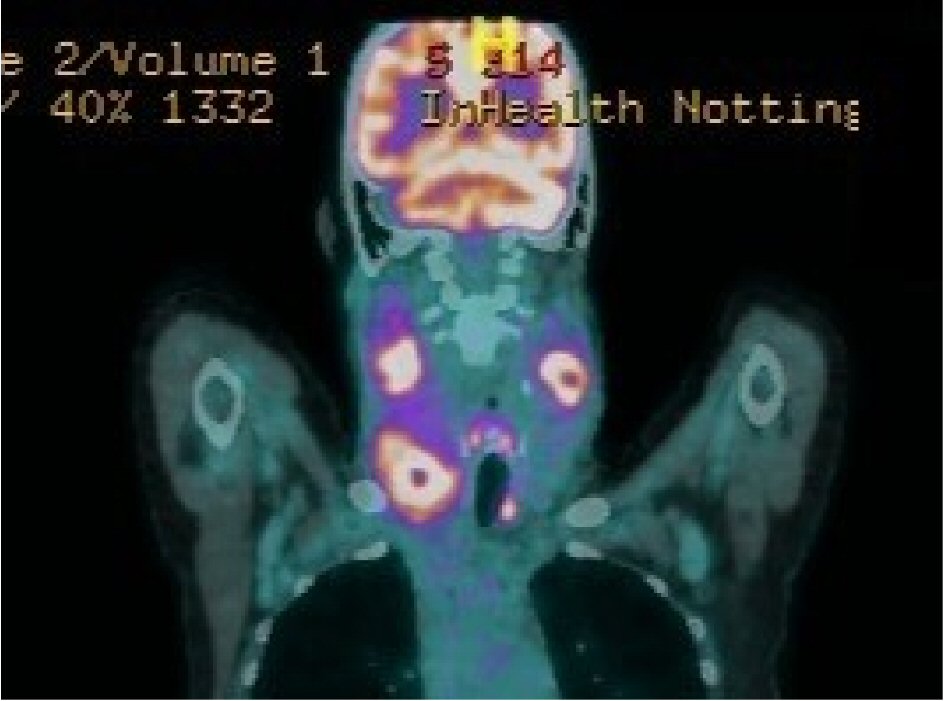 Click for large image | Figure 1. The 18FDG-PET/CT scan showing highly FDG-avid extensive confluent lymphadenopathy bilaterally in the neck. Central photopenia is suggestive of necrosis. |
However, core biopsies from the 18F-fludeoxyglucose (18FDG)-avid adenopathy showed focal areas of necrosis in addition to CLL infiltration. These necrotic areas contained ghost outlines of some spindle-shaped cells which were also seen to surround the necrotic areas. These cells contained nuclear inclusions, and occasional binucleated or multinucleated forms were noted. Immunostaining for HSV highlighted those cells with nuclear inclusions. The histological appearances were those of CLL with HSV infection (Fig. 2). There was no evidence of high-grade transformation. Polymerase chain reaction (PCR) performed on peripheral blood was negative for both HSV type 1 and type 2 DNA.
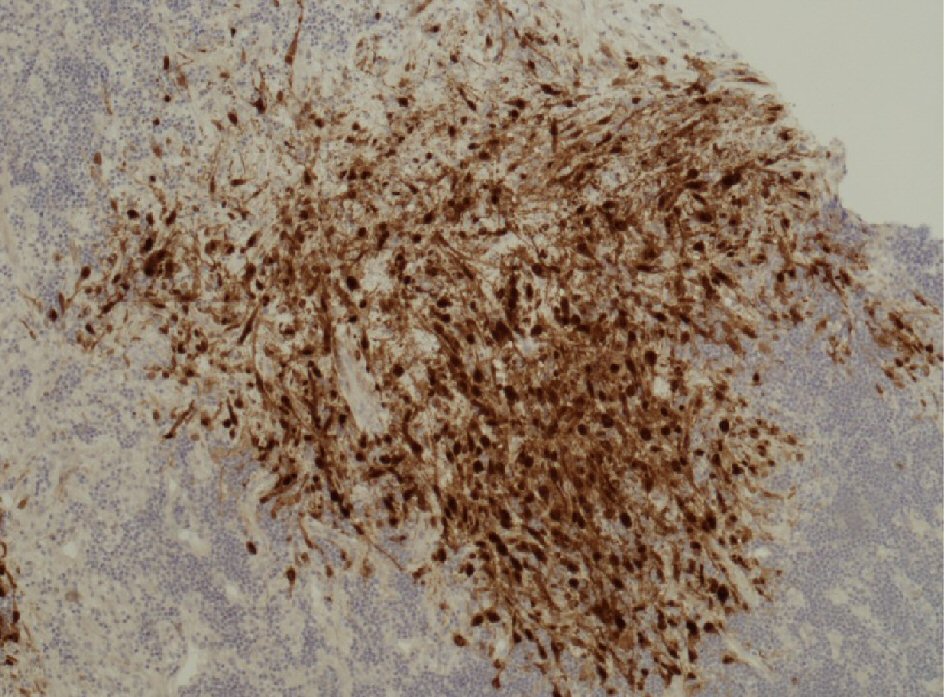 Click for large image | Figure 2. Immunostaining of adenopathy core biopsy for HSV highlighting the cells with nuclear inclusions within and surrounding the necrotic areas. |
She was treated with intravenous (IV) aciclovir (30 mg/kg/day in three divided doses) for 1 week followed by oral valaciclovir (3 g/day in three divided doses) for total of 4 weeks, together with IV immunoglobulin (IVIG) replacement therapy given pre-existing severe hypogammaglobulinemia. Clinical response was seen within 1 week of starting antiviral therapy and full resolution of neck lymphadenopathy was achieved within 4 weeks.
Treatment for CLL was subsequently indicated due to symptomatic anemia resulting from progressive bone marrow (BM) infiltration from CLL. She was treated with six cycles of bendamustine in combination with rituximab in preference to fludarabine, cyclophosphamide and rituximab (FCR) given the higher infection risk with the latter regimen [5], whilst continuing long-term aciclovir prophylaxis and IVIG given (4- weekly) to maintain a trough IgG level within the laboratory reference range. She achieved a very good partial response (BM infiltration 10-20%; > 50% nodal volume reduction; blood counts normal) and remains treatment-free and clinically well over 3 years later.
Case 2
An 81-year-old lady with treatment-naive CLL managed expectantly for the previous 5 years, presented with a progressively enlarging inguinal lymph node. There were no local or systemic signs of infection, but she had developed a mild pancytopenia and serum LDH was moderately elevated. Richter’s transformation was suspected clinically. The lymph node biopsy showed extensive necrosis with no normal lymph node architecture. At the edges of the necrotic area, there were some cells with enlarged nuclei containing intranuclear inclusions. Immunohistochemical staining for HSV showed strong positive nuclear staining for many of these cells. The pattern of necrosis and staining for HSV were consistent with HSV lymphadenitis but there was no evidence of high-grade transformation to lymphoma. Restaging CT scan showed stable nodal disease elsewhere. She was treated with 2 weeks of oral valaciclovir (3 g/day in three divided doses) and IVIG indicated for pre-existing hypogammaglobulinemia in the context of severe infection. Her CLL has not required specific therapy but she remains on long-term prophylactic aciclovir and IVIG.
Case 3
A 55-year-old man diagnosed with CLL 4 years previously was undergoing re-staging investigations with a view to initiating CLL therapy (indicated for B symptoms and progressive generalised adenopathy). He then presented acutely with a 6-day history of a rapidly enlarging, painful swelling in the right inguinal region. A tender fixed 7 × 4 cm mass was detected clinically, which was not evident on the staging CT scan performed only 2 weeks earlier. Richter’s transformation was suspected although serum LDH and calcium were both noted to be within the normal laboratory reference range. Histology from a core biopsy of the mass showed a diffuse infiltrate of small lymphoid cells with some pale nodular area of prolymphocytes and occasional immunoblasts. In addition, well-demarcated areas of necrosis were seen, with neutrophilic debris and some cells showing intranuclear inclusions, the latter staining positive for HSV immunostain. The background lymphoid population showed a typical CLL phenotype with no evidence of high-grade transformation.
On direct questioning, the patient revealed a history of genital herpes since his 20s with frequent flare-ups, the frequency of which had been increasing in recent months with the last episode only 2 weeks earlier. His peripheral blood PCR for HSV DNA was weakly positive. Immunoglobulins were normal. A diagnosis of HSV lymphadenitis was made and he was treated with IV aciclovir (30 mg/kg/day in three divided doses) for 3 days followed by oral valaciclovir (3 g/day in three divided doses) for a total of 14 days.
He responded well clinically, with resolution of the inguinal adenopathy, and was subsequently treated with FCR, achieving a complete response following six cycles of immunochemotherapy with no significant toxicities and in particular no infective episodes. He remains on prophylactic aciclovir without further reactivation of genital herpes.
| Discussion | ▴Top |
We have described three cases of progressive lymphadenopathy occurring in the context of previously untreated CLL, clinically and radiologically suspicious of Richter’s transformation, but histologically confirmed as necrotic HSV lymphadenitis. Including ours, there are total of nine such cases reported in the English-language literature so far, but we present for the first time the PET-CT appearance of this condition. These two conditions require different management approach, and yet often indistinguishable clinically with blood tests or imaging therefore represent a significant diagnostic challenge for the clinicians.
Although opportunistic infections, including those from the Herpesviridae family, are well recognized in CLL patients, this is predominantly in the context of immunosuppressive therapy and/or relapsed/refractory disease. Moreover, biopsy-proven HSV lymphadenitis is extremely rare. A study from the Mayo Clinic, Rochester examined 286 patients who had undergone lymph node biopsy for rapidly progressive lymphadenopathy from a total of 3,040 lymph node biopsies from CLL patients between 2003 and 2012, of which only three had HSV lymphadenitis [6]. CLL neoplastic B cells have been demonstrated to act as HSV antigen-presenting cells [7], and are highly sensitive to infection by HSV due to their high-level expression of herpes viral entry mediator A protein [8]. More than half of the cases reported worldwide had an underlying hematological malignancy, most commonly CLL/SLL but also mantle cell lymphoma, Hodgkin’s lymphoma, acute myeloid leukaemia (AML) and chronic myeloid leukaemia (CML) [2, 9, 10].
The diagnosis of HSV lymphadenitis in our series was not suggested by correlative clinical data, not easily distinguishable from Richter’s transformation by CT or PET-CT imaging, nor supported by sensitive PCR assay for HSV on peripheral blood. In all three cases, the diagnosis was made on histological examination of lymph node tissue including immunostaining for HSV. These cases underscore the importance of obtaining biopsy tissue in all cases of rapidly progressive or disconcordant lymphadenopathy in CLL patients, or in those with highly FDG-avid adenopathy on PET. Clearly it is therapeutically critical to distinguish HSV lymphadenitis from Richter’s transformation and a high index of clinical suspicion is essential. Prompt treatment with high-dose aciclovir and valaciclovir was successful in all three cases, supported by IVIG therapy where hypogammaglobulinemia was present. Importantly, two of our patients were subsequently able to undergo successful CLL-directed immunochemotherapy, supported with high prophylactic dose oral aciclovir therapy, without recrudescence of HSV related disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Dr. Hodgson, Dr. Jones and Dr. Fox all participated in the write up, Helen Knight provided some of the data, Dr. Sovani provided the histology image and all histology description.
| References | ▴Top |
- Klepfish A, Vaknine H, Schattner A. Never say never: unexpected herpes lymphadenitis. Lancet. 2014;384(9954):1640.
doi - Higgins JP, Warnke RA. Herpes lymphadenitis in association with chronic lymphocytic leukemia. Cancer. 1999;86(7):1210-1215.
doi - Salem A, Loghavi S, Khoury JD, Agbay RL, Jorgensen JL, Medeiros LJ. Herpes simplex infection simulating Richter transformation: a series of four cases and review of the literature. Histopathology. 2017;70(5):821-831.
doi pubmed - Pierre C, Jaubert D, Carloz E, Duval P, Boudon A. [Massive necrotizing adenitis complicating a disseminated herpes simplex virus 2 infection in chronic lymphoid leukemia]. Ann Pathol. 1991;11(1):31-35.
- Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, Lange E, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928-942.
doi - Bowen DA, Rabe KG, Schwager SM, Slager SL, Call TG, Viswanatha DS, Zent CS. Infectious lymphadenitis in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma: a rare, but important, complication. Leuk Lymphoma. 2015;56(2):311-314.
doi pubmed - Yasukawa M, Shiroguchi T, Inatsuki A, Kobayashi Y. Antigen presentation in an HLA-DR-restricted fashion by B-cell chronic lymphocytic leukemia cells. Blood. 1988;72(1):102-108.
- Eling DJ, Johnson PA, Sharma S, Tufaro F, Kipps TJ. Chronic lymphocytic leukemia B cells are highly sensitive to infection by herpes simplex virus-1 via herpesvirus-entry-mediator A. Gene Ther. 2000;7(14):1210-1216.
doi pubmed - Pilichowska ME, Smouse JH, Dorfman DM. Concurrent herpes simplex viral lymphadenitis and mantle cell lymphoma: a case report and review of the literature. Arch Pathol Lab Med. 2006;130(4):536-539.
- Cases M, Leduc C, Farmer PL, Richardson SE, Zoutman DE. Herpes simplex virus lymphadenitis: the elusive doppelganger in immunocompromised patients. Intern Med. 2014;53(21):2539-2542.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


